
Clinical outcomes and prognostic factors of parameningeal rhabdomyosarcoma in children and adolescents: results of two consecutive protocols
Highlight box
Key findings
• Among the patients with parameningeal rhabdomyosarcoma (PM-RMS), those who also had cranial base bone erosion (CBBE) or intracranial extension (ICE) had the worst outcomes. In the treatment of the PM-RMS patients with meningeal invasion (MI), the Chinese Children Cancer Group-RMS-2016 protocol produced more benefits than the Beijing Children’s Hospital-RMS-2006 protocol. Radiotherapy is important for local control, but the effect of surgery is very limited.
What is known, and what is new?
• PM-RMS is prone to central invasion and has a relatively poor prognosis. At present, the research data on PM-RMS mainly come from the Rhabdomyosarcoma (RMS) Cooperation Group in Europe and the United States, and the research data on PM-RMS in China are very limited.
• The results of our study showed that the prognosis of PM-RMS patients was not uniformly poor. Specifically, we found that patients without any MI risk factors had a relatively better prognosis than those with MI risk factors, while the prognosis of patients with coexisting CBBE and ICE, with or without cranial nerve palsy, was very poor. Further, the results showed that PM-RMS patients with MI might benefit from intensive six-drug chemotherapy combined with radiation, but the effect of surgery was very limited.
What is the implication and what should change now?
• The results of this research provide a basis for further optimizing the treatment plan for RMS in the parameningeal site.
Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and adolescents aged under 15 years, and accounts for about 50% of all soft tissue sarcomas in children (1). The parameningeal (PM) site is the most common primary site of RMS, and parameningeal rhabdomyosarcoma (PM-RMS) accounts for about 20% of all RMS cases (1). The PM site refers to the areas that occur in the middle ear, mastoid process, nasal cavity, nasopharynx, paranasal sinus, parapharyngeal area, infratemporal fossa, and pterygoid fossa, and the non-PM areas that extend to the PM area. The PM area is considered an unfavorable prognostic factor for RMS.
PM-RMS can easily progress to meningeal invasion (MI), as well as cranial base bone erosion (CBBE), cranial nerve paralysis (CNP), and intracranial extension (ICE). According to the results of some clinical trials, the prognosis of PM-RMS is affected by many factors, including the primary site, tumor size, age, MI signs, and radiotherapy (2). Our center (Beijing Children’s Hospital) first began registering its research on PM-RMS in 2013, and has been optimizing and stratifying its chemotherapy scheme for PM-RMS since 2016. This study analyzed the clinical characteristics, survival, and prognostic factors of the children and adolescents with PM-RMS enrolled in the Beijing Children’s Hospital (BCH)-RMS study in the past 10 years in an attempt to provide more research data to further optimize the treatment of these patients. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-41/rc).
Methods
Patients
We retrospectively analyzed the data of PM-RMS patients diagnosed at BCH from September 2013 to August 2021. To be eligible for inclusion in this study, the patients had to meet the following inclusion criteria: (I) be aged younger than 18 years and have a pathological diagnosis of RMS and no history of radiotherapy and chemotherapy; (II) have the PM region, which includes the middle ear, mastoid process, nasal cavity, nasopharynx, parapharyngeal region, infratemporal fossa and pterygoid fossa, and the non-PM region extending to the PM region, as the primary site; and (III) have undergone standardized treatment and follow up according to the designated diagnosis and treatment plan. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had an unclear pathological diagnosis; (II) had a history of radiotherapy and chemotherapy; (III) had refused further treatment after receiving less than two courses of chemotherapy; and/or (IV) had been lost to follow up. The patients in this group were followed up until August 31, 2022. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University (No. 2018-k-106) and informed consent was taken from all the patients’ parents or legal guardians.
Staging and risk stratification
The tumor, node, metastasis (TNM) staging system was used to determine the clinical staging of the patients, and the Intergroup Rhabdomyosarcoma Study (IRS) system was used to determine the postsurgical staging. Under the BCH-RMS-2006 protocol, the patients were allocated to one of the following three risk groups: low, intermediate, and high. Since 2016, patients with one or more MI signs were defined as the central nervous invasion (CNI) group according to the Chinese Children Cancer Group (CCCG)-RMS-2016 protocol. In PM-RMS, MI signs, including CNP, CBBE, ICE, and the presence of tumor cells in cerebrospinal fluid (CSF), need to be evaluated. CNP refers to the presence of one or more cranial nerve paralyses at the initial diagnosis, ICE refers to radiographic evidence of tumor invasion into the cranium, and CBBE refers to the presence of a tumor invading the skull base bone. A cytological examination of CSF was performed to detect tumor cells.
Treatment regimen
A comprehensive treatment regimen, including surgery, chemotherapy, and radiotherapy, was adopted. Surgery was only considered in cases in which the tumor could be completely removed without causing damage to critical organs. In the other cases, a biopsy was performed, followed by chemotherapy and then surgical intervention. Only the patients in IRS group I with embryonal RMS did not require radiotherapy; all the other cases required radiotherapy. Any regional metastatic lymph nodes required radiotherapy. The choice of radiotherapy included external radiotherapy, proton therapy and 125I particle implantation. Radiotherapy started from the 12th week of chemotherapy. In the case of central nervous system compression, radiotherapy and chemotherapy were synchronized from week 0.
Under the BCH-RMS-2006 protocol, the patients classified as having low-risk disease received vincristine, actinomycin D, and cyclophosphamide (VAC) for 24 weeks. While those classified as having medium- and high-risk disease received a randomized schedule of VAC, alternated with vincristine, topotecan, and cyclophosphamide (VTC) for 39 weeks.
The CCCG-RMS-2016 protocol (3) was first applied in 2016. Under this protocol, the patients were divided into four risk groups. The patients in the low-risk group received VAC for 12 weeks followed by vincristine and actinomycin D (VA) for 12 weeks. Those in the intermediate-risk group received VAC or VAC/vincristine and irinotecan (VI) alternation for 40 weeks. Those in the high-risk group received VAC/VI alteration, followed by vincristine, dactinomycin, and cyclophosphamide (VDC)/ifosfamide and etoposide (IE) alternation for 54 weeks. For the CNI group, a six-drug combination regimen was used, including vincristine, actinomycin D, and ifosfamide (VAI), vincristine, actinomycin D, and carboplatin (VACa), vincristine, adriamycin, and etoposide (VDE), vincristine, adriamycin, and ifosfamide (VDI) alternation for 48 weeks. The dosage of the drugs is detailed in Tables S1,S2.
Analysis of the therapeutic effects and follow up
Complete response (CR) was defined as the complete disappearance of all lesions for more than 4 weeks, and negative results for the bone marrow cell smear in patients with bone marrow metastasis. Partial response (PR) was defined as a reduction in the primary tumor of ≥64%, a reduction in the metastatic tumor of ≥30%, and no new tumors. Progression disease (PD) was defined as an increase in the primary tumor of ≥40%, an increase in the metastatic of ≥20%, or new lesions. Stable disease (SD) was defined as a tumor volume between that detailed for PD and PR.
After completing treatment, the patients underwent regular follow up once every 3 months in the first year, once every 4 months after 2–3 years, once every 6 months in the fourth year, and once a year after 5–10 years. The patients in this study were followed up until August 31, 2022.
Statistical analysis
All the statistical analyses were performed using SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The main end points of this study were 5-year overall survival (OS) and 5-year progression-free survival (PFS). Survival curves were calculated using the Kaplan-Meier method. OS was defined as the time from the beginning of treatment to death due to any reason or the last follow-up time. PFS was defined as the time from the beginning of treatment to the recurrence and progression of the disease, and the last follow-up time for event-free children. The prognostic factors were evaluated by univariate and multivariate analyses using Cox’s proportional hazards model. Statistical significance was set at P<0.05.
Results
Clinical characteristics
A total of 86 PM-RMS patients were admitted to BCH from September 2013 to August 2021. Of these 86 patients, six were excluded from the study, as they did not receive treatment, or refused treatment without any progression after receiving less than two courses of chemotherapy. Thus, a total of 80 newly diagnosed PM-RMS patients were included in the statistical analysis. Of the 80 patients, 48 (60.0%) were male and 32 (40.0%) were female. The study had a male to female ratio of 1.5:1. The median age of the patients was 64.5 months (range, 7–184 months). The clinical characteristics of the included patients are shown in Table 1.
Table 1
| Characteristics | Number, n (%) |
|---|---|
| Gender | |
| Male | 48 (60.0) |
| Female | 32 (40.0) |
| Age (years) | |
| <1 | 2 (2.5) |
| 1–2 | 14 (17.5) |
| 3–9 | 53 (66.3) |
| ≥10 | 11 (13.8) |
| Pathology type | |
| Embryonal | 44 (55.0) |
| Alveolar | 31 (38.8) |
| Other | 5 (6.3) |
| Tumor size (cm) | |
| <5 | 36 (45.0) |
| ≥5 | 44 (55.0) |
| IRS group | |
| II | 2 (2.5) |
| III | 56 (70.0) |
| IV | 22 (27.5) |
| Tumor site | |
| Nasal cavity, nasopharynx | 23 (28.8) |
| Paranasal sinus | 7 (8.8) |
| Middle ear, mastoid | 11 (13.8) |
| Infratemporal fossa/pterygoid fossa | 19 (23.8) |
| Non-parameningeal area with meningeal parameningeal extension | 20 (25.0) |
| Fusion status | |
| Negative | 44 (55.0) |
| Positive | 22 (27.5) |
| Unknown | 14 (17.5) |
| Risk factors | |
| CBBE | 64 (80.0) |
| ICE | 25 (31.3) |
| CNP | 18 (22.5) |
| CSF | 2 (2.5) |
| No risk factor | 11 (13.8) |
| Only one risk factor | 34 (42.5) |
| Two or more risk factors | 35 (43.8) |
| Lymph node metastasis | |
| N0 | 44 (55.0) |
| N1 | 36 (45.0) |
| Tumor invasion | |
| T1 | 13 (16.3) |
| T2 | 67 (83.8) |
| Treatment | |
| Surgery + chemotherapy | 3 (3.8) |
| Chemotherapy + radiotherapy | 32 (40.0) |
| Surgery + chemotherapy + radiotherapy | 43 (53.8) |
| Only chemotherapy | 2 (2.5) |
| Surgery | |
| Biopsy only | 32 (40.0) |
| R0 or R1 resection | 18 (22.5) |
| R2 resection | 30 (37.5) |
| Radiotherapy | |
| Yes | 74 (92.5) |
| No | 6 (7.5) |
| Chemotherapy response* | |
| Yes | 53 (66.3) |
| No | 27 (33.8) |
| Chemotherapy regimen | |
| BCH-RMS-2006 | 18 (22.5) |
| CCCG-RMS-2016 | 62 (77.5) |
*, achieving a CR or PR after four cycles of the chemotherapy. IRS, Intergroup Rhabdomyosarcoma Study; CBBE, cranial base bone erosion; ICE, intracranial extension; CNP, cranial nerve palsy; CSF, cerebrospinal fluid; T1, tumor confined to anatomical site of origin; T2, extension and/or fixative to surrounding tissue; BCH-RMS-2006, Beijing Children’s Hospital-Rhabdomyosarcoma-2006; CCCG, Chinese Children Cancer Group; CR, complete response; PR, partial response.
The most common primary site was the nasal cavity. Specifically, the most common primary sites were the nasal cavity and nasopharynx (23/80, 28.8%), followed by infratemporal fossa-pterygoid fossa (19/80, 23.8%), and the non-PM area with PM extension (20/80, 25.0%). A total of 69 (86.2%) patients presented with MI signs, of whom 35 (43.8%) had two or more MI signs. Among the 69 MI patients, 18 (22.5%) had CNP, 64 (80.0%) had CBBE, 25 (31.3%) had ICE, and 2 (2.5%) had CSF tumor cell positive results.
The most common pathological subtype was the embryonal type (44/80, 55.0%). The proportion of patients with a tumor diameter <5 cm (36/80, 45.0%) was slightly lower than that of patients with a tumor diameter ≥5 cm (44/80, 55.0%). Of the 80 patients, 36 (45.0%) had regional lymph node metastasis. Additionally, of the 80 patients, the FOXO1 gene fusion was negative in 44 (55.0%) patients, positive in 22 (27.5%) patients, and unknown in 14 (17.5%) patients. Further, 12 of the 80 (15.0%) patients had lung metastasis, and 3 of the 80 (3.8%) patients had bone marrow metastasis.
At the time of diagnosis, only 2 (2.5%) of the 80 patients achieved complete resection of the gross tumor (IRS group II). Of the 80 patients, 78 (97.5%) began chemotherapy after biopsy (including puncture biopsy and open biopsy), of whom 56 (70.0%) were in IRS group III and 22 (27.5%) were in IRS group IV. After induction chemotherapy, 53 of 78 (67.9%) of the patients achieved a CR or PR in imaging. Of the 80 patients, 48 (60%) received surgical treatment, but only 18 (22.5%) achieved R0 or R1 resection.
Of the 80 patients, 75 (93.8%) received radiotherapy; however, 5 (6.2%) did not receive radiotherapy due to PD. The radiotherapy dose was 44.8–50.4Gy. The radiotherapy methods included external radiotherapy (60/80, 75.0%), 125I particle implantation (8/80, 10.0%), and proton therapy (6/80, 7.5%). The timing of radiotherapy was 12–20 weeks after the start of chemotherapy.
Survival analysis
The median follow-up time of the patients in this study was 20.5 months (range, 5–100 months). The 5-year PFS and OS rates were 45.6% and 51.7%, respectively. The survival rate of the patients in this study improved over time. In this study, of the 80 patients, 18 (22.5%) were treated with the RMS-BCH-2006 protocol and 62 (77.5%) were treated with the CCCG-RMS-2016 protocol. In relation to the patients who received the old and new protocols, the 5-year PFS rates were 22.2% and 53.6%, respectively (P<0.05), while the 5-year OS rates were 33.3% and 57.0%, respectively (P<0.05). In relation to the PM-RMS patients with MI, the 5-year PFS rates were 14.3% and 51.1%, while the 5-year OS rates were 21.4% and 52.7% for those who received the old and new protocols, respectively (P<0.05) (Figure 1). In relation to the PM-RMS patients without MI, there was no significant difference in the PFS and OS rates between those who received the new and old protocols.

The survival rate of the group without MI was higher than that of the group with MI (5-year PFS: 63.6% vs. 42.4%, and 5-year OS: 71.6% vs. 48.1%), but the differences were not statistically significant. The patients with CBBE accompanied by ICE with or without CNP had the worst prognosis (Figure 2). The extent of surgical resection had no significant effect on survival.
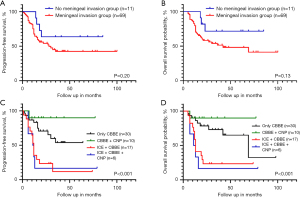
The Cox univariate analysis results are set out in Table 2. The Cox multivariate analysis results showed that the coexistence of CBBE and ICE, the absence of radiotherapy, a poor response to induction chemotherapy, and the use of the BCH-RMS-2006 protocol were risk factors affecting PFS and OS (Table 3).
Table 2
| Clinical features | 5-year OS (%) |
5-year PFS (%) |
5-year OS | 5-year PFS | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||||
| Sex (male vs. female) | 55.0 vs. 41.5 | 48.4 vs. 41.1 | 1.002 (0.505–1.987) | >0.99 | 0.857 (0.396–1.855) | 0.70 | |
| Age (years) | |||||||
| <1 | 100 | 100 | |||||
| 1–2 | 41.7 | 42.9 | 0.890 (0.333–2.379) | 0.868 (0.325–2.317) | |||
| 3–9 | 59.8 | 51.7 | 0.544 (0.238–1.245) | 0.678 (0.303–1.517) | |||
| ≥10 | 32.7 | 27.3 | 1 | 0.44 | 1 | 0.79 | |
| Pathology type | |||||||
| Embryonal | 57.0 | 47.4 | 0.408 (0.118–1.417) | 0.327 (0.096–1.112) | |||
| Alveolar | 49.3 | 46.2 | 0.554 (0.160–1.923) | 0.333 (0.096–1.159) | |||
| Others | 26.7 | 40.0 | 1 | 0.33 | 1 | 0.19 | |
| Tumor size (<5 vs. ≥5 cm) | 61.0 vs. 44.4 | 55.7 vs. 37.7 | 1.420 (0.716–2.822) | 0.32 | 1.341 (0.703–2.556) | 0.37 | |
| Lymph node metastasis (N0 vs. N1) | 60.4 vs. 41.5 | 53.3 vs. 36.4 | 0.480 (0.244–0.945) | 0.03 | 0.536 (0.284–1.010) | 0.05 | |
| IRS group | |||||||
| II | 50.0 | 50.0 | 0.778 (0.100–6.045) | 0.990 (0.129–7.600) | |||
| III | 52.7 | 48.5 | 0.801 (0.390–1.645) | 0.686 (0.351–1.343) | |||
| IV | 47.5 | 37.8 | 1 | 0.83 | 1 | 0.53 | |
| T (T1 vs. T2) | 65.9 vs. 49.0 | 52.7 vs. 44.0 | 0.475 (0.167–1.352) | 0.16 | 0.751 (0.314–1.796) | 0.52 | |
| Fusion status | |||||||
| Unknown | 21.4 | 21.4 | 3.267 (1.258–8.484) | 3.247 (1.298–8.117) | |||
| Negative | 56.6 | 44.7 | 1.415 (0.586–3.415) | 1.583 (0.696–3.599) | |||
| Positive | 63.7 | 60.2 | 1 | 0.03 | 1 | 0.03 | |
| Primary site | |||||||
| Nasal cavity, nasopharynx | 54.3 | 47.0 | 0.632 (0.268–1.494) | 0.624 (0.275–1.419) | |||
| Paranasal sinus | 68.6 | 68.6 | 0.418 (0.092–1.895) | 0.344 (0.077–1.544) | |||
| Middle ear, mastoid | 50.5 | 40.4 | 0.868 (0.301–2.504) | 0.996 (0.373–2.665) | |||
| Infratemporal fossa/pterygoid fossa | 57.7 | 51.4 | 0.584 (0.226–1.509) | 0.618 (0.252–1.516) | |||
| Non parameningeal area with meningeal invasion | 36.8 | 36.1 | 1 | 0.66 | 1 | 0.51 | |
| ICE (no vs. yes) | 63.2 vs. 28.0 | 56.1 vs. 22.4 | 0.319 (0.163–0.623) | 0.001 | 0.328 (0.174–0.618) | 0.001 | |
| CBBE (no vs. yes) | 54.1 vs. 51.0 | 49.2 vs. 44.5 | 0.760 (0.330–1.750) | 0.52 | 0.864 (0.388–1.842) | 0.67 | |
| CNP (no vs. yes) | 53.1 vs. 46.8 | 44.9 vs. 49.6 | 0.841 (0.381–1.854) | 0.67 | 1.032 (0.474–2.248) | 0.94 | |
| CSF (no vs. yes) | 48.1 vs. 0.0 | 47.1 vs. 0.0 | 0.317 (0.076–1.330) | 0.12 | 0.336 (0.080–1.406) | 0.14 | |
| Only CBBE (no vs. yes) | 44.0 vs. 65.0 | 40.4 vs. 53.9 | 1.848 (0.864–3.950) | 0.11 | 1.702 (0.847–3.422) | 0.14 | |
| CBBE + CNP (no vs. yes) | 47.7 vs. 90.0 | 40.9 vs. 90.0 | 4.816 (0.659–35.189) | 0.12 | 5.963 (0.819–43.436) | 0.08 | |
| CBBE + ICE (no vs. yes) | 60.2 vs. 23.5 | 54.2 vs. 11.8 | 0.312 (0.156–0.625) | 0.001 | 0.307 (0.158–0.595) | <0.001 | |
| CBBE + CNP + ICE (no vs. yes) | 54.5vs. 16.7 | 47.9 vs. 16.7 | 0.308 (0.118–0.804) | 0.02 | 0.345 (0.134–0.892) | 0.03 | |
| Risk factors | |||||||
| No risk factor (no vs. yes) | 48.1 vs. 71.6 | 42.4 vs. 63.6 | 0.412 (0.126–1.351) | 0.14 | 0.517 (0.183–1.458) | 0.21 | |
| One (no vs. yes) | 45.0 vs. 61.9 | 40.2 vs. 52.3 | 1.547 (0.778–3.076) | 0.21 | 1.511 (0.792–2.883) | 0.21 | |
| Two or more (no vs. yes) | 52.9 vs. 38.9 | 53.2 vs. 33.6 | 0.424 (0.217–0.831) | 0.01 | 0.471 (0.250–0.888) | 0.02 | |
| Surgery | 51.6 vs. 47.2 | 44.5 vs. 49.1 | 1.257 (0.547–2.888) | 0.59 | 1.311 (0.602–2.854) | 0.46 | |
| Only biopsy | 33.3 | 40.8 | 1.253 (0.593–2.649) | 0.72 | 1.101 (0.542–2.234) | 0.76 | |
| R0 or R1 resection | 47.2 | 49.1 | 0.894 (0.354–2.256) | 0.800 (0.339–1.890) | |||
| R2 resection | 54.0 | 47.1 | 1 | 1 | |||
| Radiotherapy (no vs. yes) | 0.0 vs. 55.8 | 0.0 vs. 49.3 | 47.518 (11.437–197.412) |
<0.001 | 34.185 (9.916–117.856) |
<0.001 | |
| Chemotherapy response* (no vs. yes) | 29.0 vs. 59.9 | 34.6 vs. 51.4 | 2.282 (1.152–4.519) | 0.02 | 2.107 (1.107–4.009) | 0.02 | |
| Chemotherapy regimen (old vs. new) | 33.3 vs. 57.0 | 22.2 vs. 53.6 | 2.095 (1.047–4.190) | 0.04 | 2.227 (1.154–4.295) | 0.02 | |
*, achieving a CR or PR after four cycles of the chemotherapy. PFS, progression-free survival; OS, overall survival; PM-RMS, parameningeal rhabdomyosarcoma; HR, hazard ratio; CI, confidence interval; IRS, Intergroup Rhabdomyosarcoma Study; T1, tumor confined to anatomical site of origin; T2, extension and/or fixative to surrounding tissue; ICE, intracranial extension; CBBE, cranial base bone erosion; CNP, cranial nerve palsy; CSF, cerebrospinal fluid; CR, complete response; PR, partial response.
Table 3
| Clinical features | 5-year OS | 5-year PFS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| CBBE + ICE (no vs. yes) | 0.191 (0.090–0.406) | <0.001 | 0.198 (0.097–0.405) | <0.001 | |
| Chemotherapy response* (no vs. yes) | 2.169 (1.015–4.632) | 0.046 | 2.195 (1.095–4.550) | 0.04 | |
| Radiotherapy (no vs. yes) | 64.351 (14.246–290.698) | <0.001 | 38.626 (10.482–142.329) | <0.001 | |
| Chemotherapy protocol (old vs. new) | 2.518 (1.232–5.147) | 0.01 | 2.618 (1.321–5.190) | 0.006 | |
*, achieving CR or PR after 4 cycles of the chemotherapy. PFS, progression-free survival; OS, overall survival; PM-RMS, parameningeal rhabdomyosarcoma; HR, hazard ratio; CI, confidence interval; CBBE, cranial base bone erosion; ICE, intracranial extension; CR, complete response; PR, partial response.
Discussion
The PM area is the most common site for RMS; it is the site for roughly 50% of head and neck RMS cases (1). Due to the special anatomical site of RMS tumors, there is a risk of central nervous system infiltration, which contributes to its poor prognosis. This study retrospectively analyzed 10 years of research data related to RMS in the PM region from our center.
Based on the clinical characteristics, 86.2% of the patients in this study had identifiable risk factors for MI. This figure is higher than that reported in the literature (of 65–80%) (2,4,5). The survival analysis data showed that the 5-year OS and PFS rates of the PM-RMS patients were 51.7% and 45.6%, respectively. These rates are lower than the OS rate reported for RMS (6). The Cox regression analysis showed that the prognosis of PM-RMS was mainly related to the extent of MI, radiotherapy, the chemotherapy protocol, and the response to early induction therapy, rather than the tumor size, IRS group, pathological subtype, and FOXO1 fusion.
PM areas are recognized as sites with a poor prognosis; however, according to several studies, the prognosis of PM-RMS patients is not uniformly poor (4,5,7,8). One study analyzed PM-RMS data from previous European and American research groups (5), including data from the IRS group III, SIOP84, CWS81, and ICS79 clinical studies, and reported that the OS rate of PM-RMS was 39–74%. The 5-year OS rates were 50–97% and 38–61% for those without risk factors for MI and those with risk factors for MI, respectively. In 2014, data collected from the United States of America and Europe (2) showed that the 5-year PFS and OS rates of PM-RMS patients were 64.9% and 69.5%, respectively. Patients without any MI signs performed best with a 5-year OS rate of 79.4%. The OS rate of patients with CNP and/or CBBE decreased to 70.9%, while the 5-year OS rate of patients with ICE decreased to 61.1%. The study also showed that the prognosis of PM-RMS patients was mainly related to age (<3 years old or >10 years old), MI, tumor diameter >5 cm, and a poor primary site of PM.
Conversely, the survival rate of the PM-RMS patients in this study was lower than the rates reported in European and American studies, and the extent of MI was related to prognosis. The prognosis of the patients with ICE (with or without CBBE/CNP) was very poor. The prognosis of patients with a single risk factor was relatively good, and the 5-year OS reached 65.0%.
Previous research has reported that the prognosis of PM-RMS patients is poor for those with RMS in the pterygoid fossa, infratemporal fossa and paranasal sinus, while the prognosis of PM-RMS patients is relatively good for those with RMS in the middle ear-mastoid process and nasal nasopharynx (2). However, the present study found no significant difference in the prognosis of patients based on the primary site. There may be a number of reasons for the difference between the results of this study and those of other research groups. The proportion of children with MI in this study was significantly higher than that reported in the literature; the proportion of children with ICE reached 30%, and the proportion of children with two or more MI signs was more than 40%, which suggests that the patients in this study had a higher extent of MI that was more difficult to treat.
A poor response to induction chemotherapy is related to the local treatment failure of embryonal PM-RMS (9). Our study also showed that response to induction chemotherapy is a factor affecting patient prognosis. In 2016, our center began to adopt a carboplatin- and ifosfamide-based six-drug-combined chemotherapy protocol for PM-RMS patients with MI, and the results showed that the 5-year OS and PFS rates improved. This suggests that PM-RMS children with MI risk factors may benefit from the combined six-drug intensive chemotherapy. However, it should be noted that a multi-disciplinary approach may be important in improving patient prognosis. Additionally further stratification is needed for patients with MI based on the severity of the central invasion.
Due to the anatomical constraints and infiltrative nature of PM-RMS, it is very difficult to obtain cancer negative margins without impeding on function. At present, definitive chemoradiation remains the standard treatment for PM-RMS, with surgery often limited to biopsy or salvage therapy for recurrent disease (10). Liu et al. (11) conducted a study with 16 children with PM-RMS or without PM-RMS who underwent skull maxillofacial resection with flap reconstruction. Recurrence, metastasis and death were reported in 7, 7, and 5 patients, respectively. However, Machavoine et al. (12) showed that lymph node surgery and secondary resection of the primary tumor of PM-RMS may improve the event free survival of alveolar RMS patients. Research from Boston Children’s Hospital and the Dana-Farber Cancer Institute revealed (13) that only 35% of PM-RMS patients received surgery, and those who were able to undergo surgery had a significantly higher 5-year survival.
In the present study, 60% of the PM-RMS patients underwent surgery, but only 22.5% of the patients achieved complete surgical resection with or without negative margins, and most of the children only underwent biopsy or partial resection. The survival analysis showed that achieving R0/R1 resection (or not) was not a prognostic factor. It is difficult to establish a standard therapeutic schedule for PM-RMS surgery, especially for advanced disease. However, there is a need to explore novel surgical interventions and provide more individualized interventions according to patients on a case-by-case basis (14).
Many studies have emphasized the absolute importance of radiotherapy in PM-RMS treatment (10,15,16), regardless of age. Among them, a retrospective study of IRS groups II–IV (17) showed that radiotherapy doses >47.5 Gy were associated with lower local recurrence rate, while hyperfractionation radiotherapy, with a higher dose, did not significantly improve patient prognosis.
The timing of radiotherapy is important (18). A study of IRS-IV and D9803 data showed that (15) radiotherapy for children with CNP and CBBE at week 12 of treatment did not affect patient prognosis, and radiotherapy was recommended at week 0 only when there was ICE. The children with ICE in this study did not receive radiotherapy at week 0, and it is not yet known whether it is a factor affecting prognosis. In early enrolled cases, 8 were treated with 125I seed implantation, 5 of whom (all with MI) died of local progression, suggesting that 125I seed implantation may not be suitable for PM-RMS children with MI risk factors. In addition, more optimized local treatments including individualized surgery strategies and advanced radiotherapy technologies, such as intensity-modulated radiation therapy and proton therapy (19), and the timing of radiotherapy are also key issues in PM-RMS treatment.
Conclusions
In conclusion, we showed that not all PM-RMS patients have a very poor prognosis. However, PM-RMS patients with MI signs have a poor prognosis, and those with ICE have an extremely poor prognosis. This study also showed that PM-RMS patients with MI could benefit from intensive chemotherapy combined with radiation therapy, and the effect of surgery was very limited.
Acknowledgments
The authors would like to thank the patients and their families for participating in the study.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-41/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-41/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-41/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University (No. 2018-k-106) and informed consent was obtained from all the patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zarrabi A, Perrin D, Kavoosi M, et al. Rhabdomyosarcoma: Current Therapy, Challenges, and Future Approaches to Treatment Strategies. Cancers (Basel) 2023;15:5269. [Crossref] [PubMed]
- Merks JH, De Salvo GL, Bergeron C, et al. Parameningeal rhabdomyosarcoma in pediatric age: results of a pooled analysis from North American and European cooperative groups. Ann Oncol 2014;25:231-6. [Crossref] [PubMed]
- Cancer Association. Subspecialty Group of Oncology, the Society of Pediatric Surgery, Chinese Medical Association. Recommendations for the diagnosis and treatment of rhabdomyosarcoma in Chinese children and adolescents(CCCG-RMS-2016). Zhonghua Er Ke Za Zhi 2017;55:724-8. [Crossref] [PubMed]
- Raney RB, Meza J, Anderson JR, et al. Treatment of children and adolescents with localized parameningeal sarcoma: experience of the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through -IV, 1978-1997. Med Pediatr Oncol 2002;38:22-32. [Crossref] [PubMed]
- Benk V, Rodary C, Donaldson SS, et al. Parameningeal rhabdomyosarcoma: results of an international workshop. Int J Radiat Oncol Biol Phys 1996;36:533-40. [Crossref] [PubMed]
- Gartrell J, Pappo A. Recent advances in understanding and managing pediatric rhabdomyosarcoma. F1000Res 2020;9:F1000 Faculty Rev-685.
- Fang Z, Duan C, Wang S, et al. Pediatric spindle cell/sclerosing rhabdomyosarcoma with FUS-TFCP2 fusion: a case report and literature review. Transl Pediatr 2024;13:178-91. [Crossref] [PubMed]
- Yang JC, Wexler LH, Meyers PA, et al. Parameningeal rhabdomyosarcoma: outcomes and opportunities. Int J Radiat Oncol Biol Phys 2013;85:e61-6. [Crossref] [PubMed]
- Ladra MM, Mandeville HC, Niemierko A, et al. Local failure in parameningeal rhabdomyosarcoma correlates with poor response to induction chemotherapy. Int J Radiat Oncol Biol Phys 2015;92:358-67. [Crossref] [PubMed]
- Casey DL, Mandeville H, Bradley JA, et al. Local control of parameningeal rhabdomyosarcoma: An expert consensus guideline from the International Soft Tissue Sarcoma Consortium (INSTRuCT). Pediatr Blood Cancer 2022;69:e29751. [Crossref] [PubMed]
- Liu Z, Zhu F, Cao W, et al. Surgical treatment of pediatric rhabdomyosarcoma in the parameningeal-nonparameningeal region. J Craniomaxillofac Surg 2020;48:75-82. [Crossref] [PubMed]
- Machavoine R, Helfre S, Bernier V, et al. Locoregional Control and Survival in Children, Adolescents, and Young Adults With Localized Head and Neck Alveolar Rhabdomyosarcoma-The French Experience. Front Pediatr 2021;9:783754. [Crossref] [PubMed]
- Dombrowski ND, Wolter NE, Robson CD, et al. Role of Surgery in Rhabdomyosarcoma of the Head and Neck in Children. Laryngoscope 2021;131:E984-92. [Crossref] [PubMed]
- Choi PJ, Iwanaga J, Tubbs RS, et al. Surgical Interventions for Advanced Parameningeal Rhabdomyosarcoma of Children and Adolescents. Cureus 2018;10:e2045. [Crossref] [PubMed]
- Spalding AC, Hawkins DS, Donaldson SS, et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: an analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys 2013;87:512-6. [Crossref] [PubMed]
- Choi Y, Lim DH. The impact of radiotherapy on clinical outcomes in parameningeal rhabdomyosarcoma. Radiat Oncol J 2016;34:290-6. [Crossref] [PubMed]
- Michalski JM, Meza J, Breneman JC, et al. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys 2004;59:1027-38. [Crossref] [PubMed]
- Lucas JT Jr, Pappo AS, Wu J, et al. Excessive Treatment Failures in Patients With Parameningeal Rhabdomyosarcoma With Reduced-dose Cyclophosphamide and Delayed Radiotherapy. J Pediatr Hematol Oncol 2018;40:387-90. [Crossref] [PubMed]
- Doyen J, Jazmati D, Geismar D, et al. Outcome and Patterns of Relapse in Childhood Parameningeal Rhabdomyosarcoma Treated With Proton Beam Therapy. Int J Radiat Oncol Biol Phys 2019;105:1043-54. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


