
Ensuring continuous access to pediatric cancer medications: insights from Brazil and East Africa
We found the study titled ‘Access to essential cancer medicines for children: a comparative analysis of availability, price, and health-system determinants in east Africa’ by Petricca et al., published in Lancet 2023 very insightful (1). It sheds light on the various barriers hindering the accessibility of childhood cancer medications within pharmaceutical value chains.
Despite Brazil’s Universal Health System (SUS), which ensures healthcare access for all, shortages in essential childhood cancer medicines persist. Throughout the entire 52-week period from January 1st, 2023, to December 31, 2023, crucial medications like asparaginase, cisplatin, fluorouracil, methotrexate (intravenous), melphalan, actinomycin D, and oxaliplatin were consistently unavailable (Figure 1).
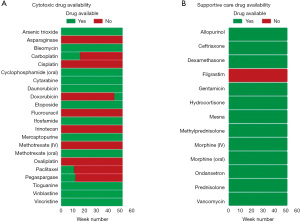
According to the regulatory body [Agência Nacional de Vigilância Sanitária (ANVISA)], registration holders are mandated to notify any discontinuation of product manufacturing, with reasons provided online. Shockingly, for the mentioned medicines, the primary reason reported by registration holders for discontinuation was ‘Commercial motivation’ (asparaginase, cisplatin, fluorouracil, and oxaliplatin), indicating a lack of interest from the registering company in marketing these drugs.
Indeed, when we calculated the median price ratios for individual medications by the ratio between the median domestic price and international buyer prices, utilizing data from the Management Sciences for Health International Medical Products Price Guide, the majority of drugs demonstrated a mean median price ratio below the internationally recognized threshold for efficient procurement (median price ratio ≤1.5) (Figure 2). Given that the data originated from 2015, adjustments were made to account for a 28.6% inflation rate in US dollars observed between 2015 and 2023. For analytical purposes, both local and Management Sciences for Health reference prices were standardized to per milligram, with local reference prices additionally converted to US dollars. Noteworthy exceptions include bleomycin, fluorouracil, and tacrolimus, each exhibiting median price ratios of 3.82, 4.68, and 2.64, respectively.

In the case of methotrexate, the reported cause was ‘Active Pharmaceutical Ingredient’, suggesting difficulties in sourcing the necessary components.
Due to Brazil’s decentralized health system, medicines are procured at the federal, state, and municipal levels. Moreover, oncological drugs are not directly allocated to treatment centers by the government. Instead, hospitals are permitted to purchase these drugs from national retailers and are subsequently reimbursed by the government. Consequently, many retailers still maintain significant stocks of medications, preventing hospitals from immediate disruptions caused by the halt in production (2). Regrettably, this is not the case for all hospitals. Since retailers are unable to acquire new drugs, even hospitals that were not initially affected will, unfortunately, face shortages in due time.
This situation echoes findings by Martei et al., demonstrating a concerning pattern where medicines become unavailable in lower-middle-income countries like Kenya and Tanzania, as well as upper-middle-income countries like Brazil (2). It underscores the urgent need for systemic solutions to ensure consistent access to essential, safe, efficacious and cost-effective medications for pediatric cancer patients as already proposed by the World Health Organization (3,4).
The reality in Brazil and in the four East African countries analyzed reflects the situation generally found in low-middle income countries (LMICs), where mortality from pediatric cancer remains disproportionately high compared to high-income countries (5). The interplay of factors such as socioeconomic status, personal income, healthcare financing and health system capacity is determinant in ensuring access to timely and high-quality care (6). Furthermore, governance impediments, including regulatory and logistical barriers, often result in poor representation of pediatric cancer medicines in national formularies, as seen in many East African countries (1). Additionally, weakly coordinated procurement and supply systems, combined with high prices by local standards, pose additional challenges to LMICs (5). As pointed out by Barr and Robertson (5) and Cotache-Condor et al. (6), improving children’s cancer care in LMICs is complex and largely driven by health system and social factors.
Paths towards a more promising scenario concerning access to pediatric cancer treatment in these countries include enhancing public health coverage, financing and structure, as well as identifying alternatives to maintain effective medicine procurement and supply management (1,5,6). The effectiveness of such measures is highlighted by the experience in Rwanda, where the development of solid referral networks and reductions in treatment expenses, led by a robust national health system infrastructure that achieves 91% of population coverage (compared to 100% in Brazil, 39,4%, 13% and 2% in Kenya, Tanzania and Uganda, respectively) (7), results in Rwanda’s low vulnerability score for childhood cancer mortality related to delays in care, despite being located in one of the world’s most vulnerable regions (8). Furthermore, regional cooperation initiatives, such as the ones developed by the Pan American Health Association (PAHO) and the East African Community, are critical for devising new strategies for resource mobilization and procurement. These initiatives aim to mitigate the challenges posed by market inequalities to the provision of effective treatment to childhood cancer patients in LMICs (8).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Pediatrics. The article has undergone external peer review.
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-55/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Petricca K, Kambugu J, Githang'a J, et al. Access to essential cancer medicines for children: a comparative mixed-methods analysis of availability, price, and health-system determinants in east Africa. Lancet Oncol 2023;24:563-76. [Crossref] [PubMed]
- Martei YM, Iwamoto K, Barr RD, et al. Shortages and price variability of essential cytotoxic medicines for treating children with cancers. BMJ Glob Health 2020;5:e003282. [Crossref] [PubMed]
- Access to NCD medicines: emergent issues during the COVID-19 pandemic and key structural factors. Geneva: World Health Organization; 2023.
- WHO Model List of Essential Medicines – 22nd List, 2021. Geneva: World Health Organization; 2021 (WHO/MHP/HPS/EML/2021.02).
- Barr R, Robertson J. Access to Cytotoxic Medicines by Children With Cancer: A Focus on Low and Middle Income Countries. Pediatr Blood Cancer 2016;63:287-91. [Crossref] [PubMed]
- Cotache-Condor C, Rice HE, Schroeder K, et al. Delays in cancer care for children in low-income and middle-income countries: development of a composite vulnerability index. Lancet Glob Health 2023;11:e505-15. [Crossref] [PubMed]
- Denburg AE, Knaul FM, Atun R, et al. Beyond the bench and the bedside: economic and health systems dimensions of global childhood cancer outcomes. Pediatr Blood Cancer 2014;61:572-6. [Crossref] [PubMed]
- Ortiz-Ospina E, Roser M. Healthcare Spending, 2017. Available online: https://ourworldindata.org/financing-healthcare


