
Automatic detection of adenoid hypertrophy on lateral nasopharyngeal radiographs of children based on deep learning
Highlight box
Key findings
• All deep learning models in this study performed well in diagnosing adenoid hypertrophy, and the DenseNet-121 model showed the best performance.
• The DenseNet121 model showed better diagnostic performance than both the junior and mid-level radiologists, achieving a diagnostic performance comparable to that of the senior radiologist.
What is known and what is new?
• Deep learning-based medical image diagnosis has demonstrated significant potential in clinical medicine.
• Deep learning methods may be feasible to diagnose adenoid hypertrophy in children using lateral nasopharyngeal radiographs.
What is the implication, and what should change now?
• The application of deep-learning to lateral nasopharyngeal radiographs could facilitate a fast, non-invasive and accurate screening for adenoid hypertrophy, which would decrease doctors’ workload and enhance clinical practices for the diagnosis and treatment of adenoid hypertrophy in children.
Introduction
As the most common cause of nasopharyngeal obstruction in pediatric patients, adenoid hypertrophy can lead to a series of disorders such as chronic open-mouth breathing, rhinitis, sinusitis, snoring, sleep apnea, daytime somnolence, and otitis media (1,2). Long-term and severe upper airway obstruction can cause structural remodeling of the pulmonary vascular bed, which can lead to pulmonary hypertension and even pulmonary heart disease, seriously affecting the growth and development of children (3). Therefore, early diagnosis and treatment of adenoid hypertrophy is of great significance in improving the prognosis of children with adenoid hypertrophy.
Lateral nasopharyngeal radiograph is the most commonly used screening tool for children with adenoid hypertrophy, and the main radiographic signs are thickening of the soft tissues in the posterior part of the nasopharynx and invasion of adenoids into the nasopharyngeal space (4,5). The adenoid/nasopharyngeal space (A/N) ratio is calculated to determine the degree of adenoid hypertrophy and nasopharyngeal obstruction, which provides the basis for targeted treatment (6). However, the accurate measurement of the thickness of adenoid and the width of nasopharyngeal cavity is highly dependent on the experience of the radiologist, therefore, with some individual variation, and lacking standardization (7,8). Inaccurate measurement of lateral nasopharyngeal radiograph may lead to incorrect assessments.
Deep learning is the “training” of artificial intelligence (AI) systems in the form of convolutional neural networks (CNNs) for fast and reliable segmentation and measurement of images, which has been proven of success in a variety of medical applications (9) such as detection of retinal layers (10), automatic measurement of spinal Cobb angle for diagnosis of spinal scoliosis (11), prediction of survival of patients with non-cellular cancer (12), and judgement of the benignness/malignancy of lung nodules with AI analysis of computed tomography (CT) images (13). We hypothesized that the deep learning method would help radiologists in diagnosing adenoid hypertrophy in children. Therefore, the present study aimed to analyze and evaluate the accuracy, reliability, and feasibility of deep learning, using lateral nasopharyngeal radiographs, in diagnosing adenoid hypertrophy in children. We present this article in accordance with the TRIPOD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-194/rc).
Methods
Subjects
Lateral nasopharyngeal X-ray images from children treated at the Department of Otolaryngology in Children’s Hospital of Soochow University, the 983th Hospital of Joint Logistics Support Forces of Chinese PLA, and Suzhou Wujiang District Children’s Hospital were retrospectively collected between January 2023 and November 2023. The inclusion criteria were: (I) clear demonstration of the nasopharyngeal airway; (II) clear demonstration of the hard palate, adenoids, skull base, and occipital slope; and (III) anonymization of all patient information. The exclusion criteria were: (I) occipital slopes that were difficult to recognize; (II) adenoids that were incompletely displayed; (III) and incorrectly positioned skulls. A dataset of 1,008 images collected from 607 males and 401 females (with an average age of 5.56±2.67 years) treated in the Children’s Hospital of Soochow University, were randomly divided into a training group (806 images) and an internal validation group (202 images) in a ratio of 4:1. One hundred eighty children from the 983th Hospital of Joint Logistics Support Forces of Chinese PLA and Suzhou Wujiang District Children’s Hospital served as the external validation group, including 98 males and 82 females, with an average age of 5.62±2.66 years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval was granted by the Ethics Committee of Children’s Hospital of Soochow (No. 2021CS146). The 983th Hospital of Joint Logistics Support Forces of Chinese PLA and Suzhou Wujiang District Children’s Hospital were informed of and agreed to the study. Individual consent for this retrospective analysis was waived.
Images collection and evaluation
Image collection
Children were placed in a standing lateral position, and instructed to inhale calmly. The digital X-ray images were captured using three X-ray machines situated in different hospitals: the DirectView DR7500 (Kodak, New York, USA), the New Oriental 1000 (Wandong, Beijing, China), and the Ysio (Siemens, Erlangen, Germany). The lateral nasopharyngeal films were collected, with tube current and tube voltage of 20–25 mA and 50–60 kV, respectively, and the filming distance of 180 cm.
Image evaluation
According to Elwany (14), an A/N ratio greater than 0.73 was considered as indicator of pathologic adenoid hypertrophy. The thickness of the adenoids in children was expressed as A and measured of the vertical length between the lowest end of the lower part of the adenoids and the lateral cranial tangent line of the occipital slope. The value of N was the width of the nasopharyngeal cavity between the posterior end of the hard palate and the base of the pterygoid plate in the nasopharyngeal cavity (Figure 1). In the present study, all plain films were manually measured by two radiologists (the junior and mid-level radiologists having 3 and 9 years of experience of imaging diagnosis, respectively) using ITK-SNAP software (version 3.8.0) to determine whether the films were labeled as “pathologic adenoid hypertrophy” (A/N ratio >0.73) or “normal adenoids” (A/N ratio ≤0.73). In case of disagreement, a senior radiologist (with more than 20 years of experience in imaging diagnosis) was consulted.

Image annotation
The images were labeled and classified using the labeling software LabelImg. The images of adenoid and nasopharyngeal airway were manually labeled using the bounding box labeling method (adenoid hypertrophy, 1; normal adenoids, 0) to indicate the selected regions (Figure 2).
Image pretreatment
The CNN models were executed in a software environment of Python3 (version 3.7.2, 64-bit), and the deep learning framework was the PyTorch library (version 1.11). The hardware included an Intel I7 12700 CPU and an NVIDIA GeForce GTX 3060 GPU.
Before training with the deep learning model, the images were pretreated, including normalization of the image intensity range and equalization of the histogram, followed by random flips, rotations and displacements of the images. The pretreatment could enhance the number of training samples for better extraction of image features and generalization of the model. Due to limited GPU resources, all the images were adjusted to 256×256 pixels. A total of 202 images randomly selected from Children’s Hospital of Soochow University were used as the internal validation group. Additionally, images of 180 children from both the 983th Hospital of Joint Logistics Support Forces of Chinese PLA and the Suzhou Wujiang District Children’s Hospital were used as the external validation group. Both internal and external validation groups were used to evaluate the performance of the models. The images in the training group were then input into the neural network for training.
Deep learning
After pretreatment, the binary classified images were provided as inputs to 5 deep learning models, i.e., AlexNet, VGG16, Inception v3, ResNet50 and DenseNet121. In the training phase, a stochastic gradient descent (SGD) optimizer was used with an initial learning rate of 0.001, a batch size of 16 and epochs of 50.
Comparation between the models and diagnosis of radiologists
In order to assess the diagnostic performance of the deep learning models with that of the radiologists, 208 images in the external validation group were visually diagnosed by the three radiologists mentioned above who independently categorized the cases as normal adenoids or adenoid hypertrophy. The differences in diagnostic ability between deep learning models and radiologists were compared using the Delong test. The Cohen Kappa test was used to assess the agreement between the deep learning models and radiologists, and between different radiologists.
Statistical analysis
Statistical analysis was performed using R version 4.1.3 software and SPSS version 26.0. Continuous variables were reported as mean (standard deviation) or median (interquartile range) and were compared using Student’s t-test or Mann-Whitney U test. Categorical variables were presented as numbers and percentages and compared using the Chi-squared test or Fisher’s exact test. A significance level of 0.05 was established to determine statistical significance. The area under the curve (AUC), accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the receiver operating characteristic (ROC) curves were used to assess the performance of each model in the training and validation groups. Kappa values were used to determine inter-observer agreement (15). Kappa values of 0.81–1.00 indicated perfect consistency, 0.61–0.80 indicated high consistency, 0.41–0.60 indicated moderate consistency, 0.21–0.40 indicated fair consistency, and 0–0.20 indicated very low consistency. P<0.05 was considered as a level with significant difference.
Results
General data
The present study collected lateral nasopharyngeal images from 1,188 children, including 705 males (mean age: 5.57±2.69 years) and 483 females (mean age: 5.56±2.62 years), with an average age of 5.57±2.66 years (ranging from 8 months to 13 years; Table 1). There was no statistically significant difference in the gender and age of the children in the three groups (P>0.05).
Table 1
| Characteristic | Training group (n=806) | Internal validation group (n=202) | External validation group (n=180) |
|---|---|---|---|
| Age (years) | 5.57±2.68 | 5.54±2.64 | 5.62±2.66 |
| Sex | |||
| Male | 486 (60.3) | 121 (59.9) | 98 (54.4) |
| Female | 320 (39.7) | 81 (40.1) | 82 (45.6) |
| Clinical evaluation | |||
| Adenoid hypertrophy | 574 (71.2) | 132 (65.3) | 106 (58.9) |
| Normal adenoid | 232 (28.8) | 70 (34.7) | 74 (41.1) |
Data are presented as n (%) or mean ± standard deviation.
Diagnostic efficacy of deep learning models
In the present study, the model that performed the best in the validation set during the training stage was selected. After 50 times of epoch, the models failed to show significant improvement in accuracy or cross-entropy loss. Figure 3 demonstrated the performance metrics of the DenseNet-121 during the network training stage in the internal and external validation groups. As the number of epochs increased, the cross-entropy loss in the validation group continued to decrease while the classification accuracy continued to rise.

The performance of the five deep learning models for diagnosing adenoid hypertrophy was compared and shown in Table 2 and Figure 4. Amongst these 5 deep models, DenseNet-121 performed better, having accuracy of 0.895 and 0.878, sensitivity of 0.870 and 0.838, specificity of 0.913 and 0.906, PPVs of 0.883 and 0.861, NPVs of 0.903 and 0.889, in the internal and external validation groups, respectively. The diagnostic performance of Inception V3 model was slightly higher than that of DenseNet-121 model in the internal validation group, having accuracy of 0.898, sensitivity of 0.873, specificity of 0.917, PPV of 0.889, and NPV of 0.905. However, the AUC of the Inception V3 model in the external validation group was 0.848, which was relatively low. ResNet50 model also showed good performance, with more consistent performance in the internal and external validation groups, with AUCs of 0.871 and 0.865, respectively. The diagnostic performance of AlexNet and VGG16 models was at average level, with AUCs of 0.839 and 0.815 in the internal validation group, and AUCs of 0.807 and 0.779 in the external validation group, respectively. Overall, all five deep learning models performed well in recognizing adenoid hypertrophy, and the DenseNet-121 model showed the best performance. The confusion matrices in three groups were shown in Figure 5.
Table 2
| Group | Model | AUC | 95% CI | ACC | SEN | SPE | PPV | NPV | P value |
|---|---|---|---|---|---|---|---|---|---|
| Training group | AlexNet | 0.857 | 0.808–0.906 | 0.856 | 0.862 | 0.852 | 0.815 | 0.891 | 0.021 |
| VGG16 | 0.844 | 0.795–0.893 | 0.837 | 0.897 | 0.791 | 0.765 | 0.910 | <0.001 | |
| Inception V3 | 0.865 | 0.816–0.913 | 0.871 | 0.816 | 0.913 | 0.877 | 0.868 | 0.020 | |
| ResNet50 | 0.896 | 0.854–0.937 | 0.891 | 0.931 | 0.861 | 0.835 | 0.943 | 0.353 | |
| DenseNet121 | 0.913 | 0.875–0.952 | 0.911 | 0.931 | 0.896 | 0.871 | 0.945 | Reference | |
| Internal validation group | AlexNet | 0.839 | 0.813–0.864 | 0.845 | 0.793 | 0.885 | 0.838 | 0.849 | 0.001 |
| VGG16 | 0.815 | 0.788–0.842 | 0.819 | 0.787 | 0.843 | 0.791 | 0.839 | <0.001 | |
| Inception V3 | 0.895 | 0.874–0.917 | 0.898 | 0.873 | 0.917 | 0.889 | 0.905 | 0.802 | |
| ResNet50 | 0.871 | 0.847–0.894 | 0.876 | 0.716 | 0.833 | 0.873 | 0.878 | 0.172 | |
| DenseNet121 | 0.892 | 0.870–0.913 | 0.895 | 0.870 | 0.913 | 0.883 | 0.903 | Reference | |
| External validation group | AlexNet | 0.807 | 0.750–0.865 | 0.833 | 0.662 | 0.953 | 0.907 | 0.802 | 0.022 |
| VGG16 | 0.779 | 0.721–0.837 | 0.817 | 0.568 | 0.991 | 0.977 | 0.766 | 0.002 | |
| Inception V3 | 0.848 | 0.794–0.902 | 0.867 | 0.743 | 0.953 | 0.917 | 0.842 | 0.377 | |
| ResNet50 | 0.865 | 0.813–0.917 | 0.872 | 0.824 | 0.906 | 0.859 | 0.881 | 0.808 | |
| DenseNet121 | 0.872 | 0.821–0.922 | 0.878 | 0.838 | 0.906 | 0.861 | 0.889 | Reference |
AUC, area under curve; CI, confidence interval; ACC, accuracy; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value.
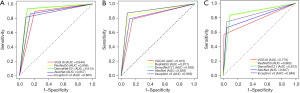

Comparison between models and radiologists
In this study, the deep learning model showing the best performance, DenseNet121, was selected for comparison with the radiologists’ diagnosis (Table 3). Although Delong’s test showed no significant difference between DenseNet121 and each radiologist in the validation group (P=0.24, P=0.52, P=0.79), DenseNet121 demonstrated higher AUC and sensitivity than that of the junior radiologist and the mid-level radiologist, and same sensitivity with the senior radiologist. Moreover, the kappa value of the diagnostic consistency between DenseNet121 and the senior radiologist was as high as 0.89.
Table 3
| Model/radiologists | AUC | 95% CI | ACC | SEN | SPE | PPV | NPV | P value |
|---|---|---|---|---|---|---|---|---|
| DenseNet121 | 0.892 | 0.870–0.913 | 0.895 | 0.870 | 0.913 | 0.883 | 0.903 | Reference |
| Junior radiologist | 0.836 | 0.742–0.930 | 0.911 | 0.739 | 0.919 | 0.765 | 0.938 | 0.24 |
| Mid-level radiologist | 0.869 | 0.781–0.956 | 0.935 | 0.783 | 0.955 | 0.692 | 0.972 | 0.52 |
| Senior radiologist | 0.901 | 0.829–0.974 | 0.926 | 0.870 | 0.933 | 0.793 | 0.963 | 0.79 |
AUC, area under curve; CI, confidence interval; ACC, accuracy; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value.
The Cohen Kappa test was used to determine the consistency among radiologists. The result showed that the Kappa value between the junior radiologist and the mid-level radiologist was 0.89, indicating perfect agreement. The Kappa value between the junior radiologist and the senior radiologist was approximately 0.76, indicating high agreement. The Kappa value between the mid-level radiologist and the senior radiologist was 0.81, indicating perfect agreement.
Discussion
Adenoid hypertrophy is an important cause of sinusitis and otitis media in children worldwide (16). In severe instances, it can result in obstructive sleep apnea (OSA) and have adverse effects on children’s growth and development (17). While nasal endoscopy is currently considered the gold standard for diagnosing adenoid hypertrophy, its invasive nature poses risks such as bleeding, pain, and infection (4,18). Conversely, X-ray radiography offers a simple, economical, and non-invasive procedure for the diagnosis of adenoid hypertrophy (19). Many studies have demonstrated the high reliability of lateral nasopharyngeal radiographs in detecting adenoid hypertrophy (20,21). Therefore, this study proposes a practical and simplified deep learning model, DenseNet121, based on lateral nasopharyngeal radiographs. This model was capable of detecting adenoid hypertrophy with high accuracy that was close to the level of senior radiologist, and was externally validated at an independent center. These results indicated that the deep learning DenseNet121 model was a reliable screening tool for adenoid hypertrophy.
Currently, there are rare studies using deep learning models to assess adenoid hypertrophy. Two previous studies applied deep learning to label the four key-points of the A/N ratio for automatic diagnosis of adenoid hypertrophy by transforming image classification task into key-point detection task (22,23). Both of these studies require manual labeling of the four key-points of the A/N ratio on lateral nasopharyngeal radiographs, have the potential possibility of general errors and are time-consuming to correct manually.
In the present study, the images were pre-processed by cropping the adenoids and nasopharyngeal airway regions from lateral nasopharyngeal radiographs using LabelImg software. During image pre-processing, the method based on region of interest (ROI) is commonly used for model construction because of these following advantages. Firstly, it is easy to crop the region on the lateral nasopharyngeal image, while the target key-point detection method may have potential errors (24). Secondly, deep learning is a method that consumes a lot of memory and computational resources (25). Inputting high-resolution images requires more computational effort and memory resources. A smaller ROI provides enough information for evaluation with fewer data inputs, which can effectively reduce the memory utilization.
Liu et al. (26) proposed a deep learning model VGG-Lite, using 1,023 lateral nasopharyngeal radiograph images for detection of adenoid hypertrophy. The VGG-Lite model showed good performance with AUC of 0.946, sensitivity of 0.898, and specificity of 0.882. In the present study, five deep learning models for diagnosis of adenoid hypertrophy were developed and evaluated using lateral nasopharyngeal X-rays, and all achieved good performance. Notably, among these models, DenseNet121 model demonstrated the best performance, with AUCs of 0.892 and 0.872 in the internal and external validation groups, respectively. The possible reason for this is that the dense architecture in DenseNet connects all the features of previous layers, which enhances the reuse of features and can improve the performance of recognizing adenoidal hypertrophy (27). However, the performance of this model still does not exceed the model proposed by Liu et al. (26). The possible reason is that all the models in the present study are pre-trained on ImageNet, which results in that pre-trained models on natural images do not reach the best performance when applied to medical images (28); whereas Liu et al. further optimized the traditional VGG model.
In addition, the present study compared the efficacy in diagnosis of adenoid hypertrophy between deep learning models and radiologists with different seniority. The results showed that the DenseNet121 model had the same sensitivity and slightly lower accuracy and specificity than that of the senior radiologist, which was generally close to the level of the senior radiologist. However, the diagnostic performance of DenseNet121 model was not significantly different from that of both the junior and mid-level radiologists, which may be due to the small amount of data in the external validation group. Nonetheless, we still believe that deep learning models may be helpful for radiologists, especially for junior radiologists, to diagnose adenoid hypertrophy.
There are some limitations in the present study. First, because of the poor cooperation of infants and young children, it was difficult to obtain standardized and accurate lateral nasopharyngeal radiographs, which may affect the accuracy in assessing adenoid hypertrophy and the measurement and calculation of the A/N ratio. Second, the model developed in the present study did not enroll the clinical factors of the children with adenoid hypertrophy. Combination of clinical variations with the model may be possible to further improve the performance of the models and obtain a more accurate diagnosis. Finally, the present study only focused on the diagnosis of adenoid hypertrophy based on lateral nasopharyngeal radiographs. In the future, we will further explore the ability of deep learning in determining the grade of adenoid hypertrophy to achieve accurate diagnosis of adenoid hypertrophy.
Conclusions
In the present study, deep learning with different convolutional network models was applied for diagnosis of adenoid hypertrophy using lateral nasopharyngeal radiographs from children. The results showed that all models exhibited good performance though with different variations, with DenseNet121 model being the best, having closely level of the senior radiologist. The study suggested that deep learning models possess the clinical value for reducing the workload in screening children with adenoidal hypertrophy and for diagnosis and medical intervention in the early stages of adenoidal hypertrophy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-194/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-194/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-194/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-194/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval was granted by the Ethics Committee of Children’s Hospital of Soochow (No. 2021CS146). The 983th Hospital of Joint Logistics Support Forces of Chinese PLA and Suzhou Wujiang District Children’s Hospital were informed of and agreed to the study. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ahmad Z, Krüger K, Lautermann J, et al. Adenoid hypertrophy-diagnosis and treatment: the new S2k guideline. HNO 2023;71:67-72. [Crossref] [PubMed]
- Calvo-Henriquez C. Coblator adenoidectomy in pediatric patients: a state-of-the-art review. Eur Arch Otorhinolaryngol 2023;280:4339-49. [Crossref] [PubMed]
- Kong F, Sun YL, Yuan B. Prevalence of pulmonary hypertension in children with adenoid or adenotonsillar hypertrophy: A meta-analysis. Int J Pediatr Otorhinolaryngol 2022;153:111019. [Crossref] [PubMed]
- Zwierz A, Masna K, Domagalski K, et al. 150th Anniversary of global adenoid investigations: unanswered questions and unsolved problems. Front Pediatr 2023;11:1179218. [Crossref] [PubMed]
- Mlynarek A, Tewfik MA, Hagr A, et al. Lateral neck radiography versus direct video rhinoscopy in assessing adenoid size. J Otolaryngol 2004;33:360-5. [Crossref] [PubMed]
- Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol 1979;133:401-4. [Crossref] [PubMed]
- Aboudara C, Nielsen I, Huang JC, et al. Comparison of airway space with conventional lateral headfilms and 3-dimensional reconstruction from cone-beam computed tomography. Am J Orthod Dentofacial Orthop 2009;135:468-79. [Crossref] [PubMed]
- Major MP, Flores-Mir C, Major PW. Assessment of lateral cephalometric diagnosis of adenoid hypertrophy and posterior upper airway obstruction: a systematic review. Am J Orthod Dentofacial Orthop 2006;130:700-8. [Crossref] [PubMed]
- Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal 2017;42:60-88. [Crossref] [PubMed]
- Zhang K, Liu X, Xu J, et al. Deep-learning models for the detection and incidence prediction of chronic kidney disease and type 2 diabetes from retinal fundus images. Nat Biomed Eng 2021;5:533-45. [Crossref] [PubMed]
- Jin C, Wang S, Yang G, et al. A Review of the Methods on Cobb Angle Measurements for Spinal Curvature. Sensors (Basel) 2022;22:3258. [Crossref] [PubMed]
- She Y, Jin Z, Wu J, et al. Development and Validation of a Deep Learning Model for Non-Small Cell Lung Cancer Survival. JAMA Netw Open 2020;3:e205842. [Crossref] [PubMed]
- Wang X, Gao M, Xie J, et al. Development, Validation, and Comparison of Image-Based, Clinical Feature-Based and Fusion Artificial Intelligence Diagnostic Models in Differentiating Benign and Malignant Pulmonary Ground-Glass Nodules. Front Oncol 2022;12:892890. [Crossref] [PubMed]
- Elwany S. The adenoidal-nasopharyngeal ratio (AN ratio). Its validity in selecting children for adenoidectomy. J Laryngol Otol 1987;101:569-73. [Crossref] [PubMed]
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360-3.
- Lam DJ, Krane NA, Mitchell RB. Relationship between Drug-Induced Sleep Endoscopy Findings, Tonsil Size, and Polysomnographic Outcomes of Adenotonsillectomy in Children. Otolaryngol Head Neck Surg 2019;161:507-13. [Crossref] [PubMed]
- Williamson A, Coutras SW, Carr MM. Sleep Endoscopy Findings in Children With Obstructive Sleep Apnea and Small Tonsils. Ann Otol Rhinol Laryngol 2022;131:851-8. [Crossref] [PubMed]
- Zwierz A, Domagalski K, Masna K, et al. Effectiveness of Evaluation of Adenoid Hypertrophy in Children by Flexible Nasopharyngoscopy Examination (FNE), Proposed Schema of Frequency of Examination: Cohort Study. Diagnostics (Basel) 2022;12:1734. [Crossref] [PubMed]
- Feres MF, Hermann JS, Cappellette M Jr, et al. Lateral X-ray view of the skull for the diagnosis of adenoid hypertrophy: a systematic review. Int J Pediatr Otorhinolaryngol 2011;75:1-11. [Crossref] [PubMed]
- Peedikakkal NT, Prakash DRS, Chandrakiran C, et al. Endoscopic Grading, Radiological Grading and Clinical Features in Children with Chronic Adenoid Hypertrophy: A Correlational Study. Indian J Otolaryngol Head Neck Surg 2023;75:725-31. [Crossref] [PubMed]
- Adedeji TO, Amusa YB, Aremu AA. Correlation between adenoidal nasopharyngeal ratio and symptoms of enlarged adenoids in children with adenoidal hypertrophy. Afr J Paediatr Surg 2016;13:14-9. [Crossref] [PubMed]
- Shen Y, Li X, Liang X, et al. A deep-learning-based approach for adenoid hypertrophy diagnosis. Med Phys 2020;47:2171-81. [Crossref] [PubMed]
- Zhao T, Zhou J, Yan J, et al. Automated Adenoid Hypertrophy Assessment with Lateral Cephalometry in Children Based on Artificial Intelligence. Diagnostics (Basel) 2021;11:1386. [Crossref] [PubMed]
- Grogger P, Sacher C, Weber S, et al. Identification of 'Point A' as the prevalent source of error in cephalometric analysis of lateral radiographs. Int J Oral Maxillofac Surg 2018;47:1322-9. [Crossref] [PubMed]
- Ruan X, Murphy RF. Evaluation of methods for generative modeling of cell and nuclear shape. Bioinformatics 2019;35:2475-85. [Crossref] [PubMed]
- Liu JL, Li SH, Cai YM, et al. Automated Radiographic Evaluation of Adenoid Hypertrophy Based on VGG-Lite. J Dent Res 2021;100:1337-43. [Crossref] [PubMed]
- Huang G, Liu Z, Van Der Maaten L, et al. Densely Connected Convolutional Networks. 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, 2017:2261-9.
- Xie J, Shi H, Du C, et al. Dual-Branch Convolutional Neural Network Based on Ultrasound Imaging in the Early Prediction of Neoadjuvant Chemotherapy Response in Patients With Locally Advanced Breast Cancer. Front Oncol 2022;12:812463. [Crossref] [PubMed]


