
Advances in magnetic resonance imaging for the assessment of paediatric focal epilepsy: a narrative review
Introduction
Background
Epilepsy is one of the most prevalent neurological disorders worldwide, affecting approximately 50 million people (1). Epilepsy affects 0.5% to 1% of children and its prevalence increases with age, with peaks at 5–9 years (2,3). It is characterised by recurrent unprovoked seizures, originating from an abnormal epileptic network in the brain which can be observed with electroencephalographic (EEG) changes. Approximately 30–40% of patients with epilepsy do not achieve seizure freedom with anti-seizure medication (4), necessitating alternative therapeutic options such as surgical resection of the epileptogenic zone. Surgical intervention in paediatric patients has been shown to increase the rate of seizure freedom and improve quality of life (5).
The success of surgical procedures presupposes the presence of a lesion identifiable on imaging. The identification of epileptogenic lesions presents a significant challenge, notably in cases classified as “magnetic resonance imaging (MRI)-negative drug resistant epilepsy (DRE)” or “non-lesional MRI” (6). This terminology can be misleading as it includes patients without visible MRI lesions (true negative) and those with subtle lesions that have a histopathological basis but are not detected through MRI (false negative). An international survey (7) revealed that MRI shows a definite lesion in 77% of patients, a subtle or suspected lesion in 6%, and no lesion in 17% of cases. Additionally, a systematic review and meta-analysis (8) reported that the overall prevalence of patients with non-lesional (NL) epilepsy in all surgical studies was 26%. The occurrence of false-positive and false-negative MRIs poses a risk of misdirecting clinical decision-making, such as surgical candidacy, the need for invasive procedures like stereo-EEG (sEEG), and “pseudo-resistance phenomena” due to misdiagnosis. The unique challenges in the diagnosis of subtle epileptogenic foci are further amplified by the ongoing process of myelination during the first years of life (9-11).
Malformations of cortical development (MCDs) represent a spectrum of common epileptogenic lesions in the paediatric population (12). Focal cortical dysplasia (FCD), the most common subtype (13), can be challenging to detect, and it may require a multidisciplinary/multi-technique approach: video-EEG, MRI with dedicated epilepsy protocols, positron emission tomography (PET) scans, and sEEG alongside a detailed integration of clinical insights is pivotal for precise localization of the lesion (14-16).
Although our review focuses on focal epilepsies, it is important to recognize that some epilepsies are not caused by a focal lesion, such as certain genetic syndromes. These can be associated with anatomical abnormalities visible on standard MRI or network anomalies that may not be detectable with current imaging techniques. Epilepsy is considered a network disorder, and imaging techniques are just one of the tools useful for medical management, presurgical planning, and post-surgical monitoring (17). Even without visible lesions, surgery can be supportive: disconnective surgery, such as posterior quadrant disconnection using a temporo-occipital-parietal approach, hemispherotomy, and corpus callosotomy, can help reduce the spread of abnormal electrical discharges and thus mitigate the effects of seizures. Other techniques include palliative approaches, such as vagus nerve stimulation and deep brain stimulation (18).
An expert consensus (19) evaluated several diagnostic tests across various centres, categorising them into universally employed tests (scalp-EEG, MRI), ancillary tests such as 3D EEG or magnetoencephalography (MEG) source imaging, fluorodeoxyglucose-PET (FDG-PET), ictal single-photon emission computed tomography (SPECT), electrocorticography (ECoG), and extra-operative invasive EEG monitoring (IEM). Additionally, there are tests with potential utility but limited data or general use experience in paediatrics, including magnetic resonance spectroscopy (MRS), EEG-triggered functional MRI (fMRI), MRI post-processing techniques, newer PET ligands, diffusion-weighted imaging (DWI) with tractography, transcranial magnetic stimulation, and intraoperative ultrasound. The authors conclude that standardizing the presurgical evaluation in children is necessary to avoid practices that increase costs and risks without documented benefit.
All these tools are essential for the accurate and effective management of patients with epilepsy. A thorough understanding of the various MRI techniques can aid paediatricians and other specialists in the multidisciplinary approach, which is crucial.
Rational and knowledge gap
There is a focus on developing more advanced MRI sequences, post-processing methods and other imaging techniques to increase the diagnostic yield in patients with MRI-negative DRE. In 2019, the International League Against Epilepsy (ILAE) proposed the Harmonised Neuroimaging of Epilepsy Structural Sequences (HARNESS) protocol, to improve the detection of epileptogenic lesions (14). The protocol consists of high-resolution 3D T1-weighted and fluid-attenuated inversion recovery sequences, the most used being magnetization-prepared rapid gradient echo (MPRAGE) and fluid attenuated inversion recovery (FLAIR), and a high in-plane resolution 2D coronal T2-weighted sequence perpendicular to the hippocampal long axis. The protocol has been proven to be both superior in detection of lesions and cost-effectiveness, although prospective studies on larger cohorts of patients are still needed (15,16).
Objective
The aim of this paper is to provide a narrative review for paediatricians/neuropaediatricians, neurologists and neurosurgeons, on advanced diagnostic techniques for the identification of focal epileptogenic lesions. We present this article in accordance with the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-166/rc).
Methods
Search strategy
The search strategy was conducted from 01 January 2024 to 31 January 2024, and it was focused on identifying relevant literature in the field of paediatric epilepsy, with a specific emphasis on advanced MRI sequences and post-processing techniques, focal epilepsy, and the application of artificial intelligence (AI) in diagnostic processes (see Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | From 1 January 2024 to 31 January 2024 |
| Database | PubMed |
| Search terms used | Focal epilepsy |
| MRI OR magnetic resonance images | |
| AI OR artificial intelligence | |
| Timeframe | No limits |
| Inclusion and exclusion criteria | Inclusion criteria: |
| • Studies including patients with patients aged 16 years or younger | |
| • Studies focused on focal epilepsy | |
| • Studies using MRI sequences | |
| • Studies published in the English language | |
| Exclusion criteria: | |
| • Studies not including patients who are 16 years old or younger | |
| • Studies using nuclear medicine imaging | |
| • Studies investigating genetic cause of epilepsy | |
| Selection process | L.V.P. and F.D.A. jointly selected the papers for the literature review. L.V.P. conducted the analysis of the selected papers, while F.D.A. provided supervision throughout the process |
MRI, magnetic resonance imaging.
Databases and keywords
The primary database utilised for the search was PubMed. The following search string was used: Focal epilepsy AND (MRI OR Magnetic Resonance Images) AND (AI OR Artificial intelligence).
We excluded the literature on nuclear medicine techniques, which was outside the scope of this review, despite their recognized diagnostic and cost-effectiveness value in epilepsy management (13,17).
Discussion
In addition to conventional MRI sequences, such as 3D T2-weighted images, FLAIR and MPRAGE, several other sequences have been utilised to improve the detection of focal epileptogenic lesions and/or for pre-surgical evaluation.
For ease of reference and to enhance the clarity of the discussion, the technical aspects of each imaging technique have been summarized in a comprehensive table (Table 2).
Table 2
| Imaging technique | Principle | Usefulness | Limitations | Clinical applicability | Patients’ age (youngest) |
|---|---|---|---|---|---|
| DWI | Measures diffusion of water molecules | Can help differentiate aggressive tumours (increased diffusion restriction) from low-grade and MCD | Susceptible to motion artefacts, low spatial resolution | Primarily used for detecting acute stroke | Not reported |
| DTI | Measures directional diffusion of water molecules | Detailed visualisation of white matter tracts, additional information on the microstructures of the brain | Possible incorrect representation of neural paths in regions with dense fibre populations; cannot distinguish afferent from efferent projections | Evaluation of the white matter networks before and after surgery | Less than 1 year old |
| DKI | Measures non-uniform (non-Gaussian) diffusion of water molecules in complex tissue | More accurate model of diffusion and improved white matter characterization | Relatively long-time acquisition, reproducibility | Detecting subtle microstructural abnormalities, such as in mesial temporal sclerosis | 5 years old |
| NODDI | Measures the orientation and density of neurites | Contribution of tissue components individually (intraneurite water, extraneurite water and cerebrospinal fluid) | Requires advanced processing techniques, longer acquisition times | Better understanding of neural architecture in epilepsy | 6 years old |
| DIR | Uses dual inversion pulses to null signals from CSF and white matter | Improved grey matter lesion detection, enhances visibility of cortical structures | Prolonged imaging times, sequence-specific artefacts | Visualising cortical lesions and grey matter abnormalities | 2 years old |
| MP2RAGE | Combines two gradient echoes to obtain a T1-weighted imaging with reduced bias | Higher resolution | More suited for 3 T or higher field scanners | Useful in detecting subtle brain lesions and anatomical details | 7 years old |
| EDGE | Adaptation of MPRAGE/MP2RAGE | Higher contrast ratio for the subcortical regions | Susceptible to artefacts, requires precise parameter settings | Enhancing visibility of lesion margins and anatomical structures in epilepsy | 14 years old |
| FLAWS | Similar to DIR, uses two inversion times of MP2RAGE to suppress CSF and white matter | Better grey matter visualisation at high magnetic field |
Long acquisition time | Improving contrast in cortical regions for cortical/subcortical lesions | 8 years old |
| ASL | Uses magnetically labelled arterial blood as an endogenous tracer | Non-invasive measurement of cerebral blood flow, no radiation exposures | Sensitive to delayed blood flow, age-related CBF changes | Assessing cerebral perfusion and identifying perfusion abnormalities | Less than 1 year old |
| QSM | Measures magnetic susceptibility to quantify tissue properties | Quantifies concentrations of iron, calcium, and other substances; improves detection of microbleeds and calcifications | Susceptible to artefacts from air-tissue interfaces, requires complex post-processing | Identification of epileptogenic tubers in TSC; offers a valuable tool for the detailed assessment of mineral deposits and tissue composition | 2 years old |
| MRS | Measures the concentration of metabolites in tissues | Provides metabolic information, can detect abnormalities in neuronal health, energy metabolism, and membrane turnover | Lower spatial resolution compared to structural MRI, susceptible to motion artefacts, complex data interpretation | Detecting and characterising metabolic changes in epilepsy, characterising tumours | Less than 1 year old |
| MRF | Generate quantitative tissue properties | High reproducibility, more objective evaluation of different tissues | Requires advanced softwares, long acquisition time | Quantitative assessment and comparison of tissue properties | 6 years old |
| MTI | Measures the exchange of magnetization between free water and macromolecular-bound water | Enhances contrast between normal and pathological tissues, useful in detecting demyelination | Sensitive to radiofrequency field inhomogeneities, longer scan times | Primarily utilised in multiple sclerosis, it has also shown good sensitivity in identifying MCD | Less than 1 year old |
| VBM, including MAP |
Statistical analysis of differences in brain anatomy using voxel-wise comparison | Identifies brain volume changes | Requires large sample sizes for statistical power, sensitive to preprocessing steps | Detecting and quantifying grey matter atrophy, useful in epilepsy research and diagnosis | 6 years old |
| SBM | Analyses cortical thickness, surface area, and volume | Detailed analysis of cortical morphology, sensitive to subtle changes in cortical structure | Requires high-quality images, sensitive to preprocessing steps | Primarily used in studying disorders like autism and schizophrenia, its application in epilepsy research is relatively limited | 1 year old |
| fMRI | Measures brain activity by detecting changes in blood flow (BOLD) | Maps brain function, identifies active brain regions during tasks | Susceptible to motion artifacts and patient compliance | Assessing brain functions, identifying seizure focus, presurgical mapping in epilepsy patients | 6 years old |
| AI | Uses machine learning algorithms to analyse imaging data | Enhance diagnostic accuracy, improve efficiency, reduce workload | Dependence on large datasets, need rigorous validation | Automated detection of abnormalities, surgical outcome prediction, improving diagnostic accuracy in epilepsy | 3 years old |
DWI, diffusion-weighted imaging; DTI, diffusion tensor imaging; DKI, diffusion kurtosis imaging; NODDI, neurite orientation dispersion and density imaging; DIR, double inversion recovery; MP2RAGE, magnetization prepared 2 rapid gradient echo; EDGE, edge-enhancing gradient echo; FLAWS, fluid and white matter suppression; ASL, arterial spin labeling; QSM, quantitative susceptibility mapping; MRS, magnetic resonance spectroscopy; MRF, magnetic resonance fingerprinting; MTI, magnetization transfer imaging; VBM, voxel-based morphometry; MAP, morphometric analysis program; SBM, surface-based morphometry; fMRI, functional magnetic resonance imaging; AI, artificial intelligence; CSF, cerebrospinal fluid; BOLD, blood oxygenation-level dependent; MCD, malformations of cortical development; CBF, cerebral blood flow.
MRI sequences
DWI highlights variations in the mobility of water protons within biological tissues. In these images, structures with “normal” diffusion appear darker due to the attenuation of the MRI signal, while areas with restricted diffusion, such as those affected by ischemia, appear brighter. This contrast helps in identifying regions with altered water molecule mobility. Together with diffusion tensor imaging (DTI) and their reconstructed parameters such as apparent diffusion coefficient (ADC), fractional anisotropy (FA), fibre tractography (FT) and recent advanced techniques such as diffusion kurtosis imaging (DKI), neurite orientation dispersion and density imaging (NODDI) and diffusion spectrum imaging (DSI), they have been studied for the identification of the epileptogenic lesion, aetiology, and preoperative evaluation. DWI and its reconstructed maps can be used to better characterise the nature of the lesion (for instance aggressive paediatric tumours will show diffusion restriction as opposed to FCD or low-grade epileptogenic tumours) while DTI is used for the evaluation of the white matter (WM) networks (motor function, visual field, and language) before and after surgery (20). DTI may also serve as a non-invasive biomarker for characterising FCD lesions (21). Tractography can be useful for characterising WM tract organisation in polymicrogyria (PMG) and lissencephaly, demonstrating that brain structural abnormalities in MCD extend beyond grey matter (GM) involvement (22).
DKI, unlike standard DTI which assumes water diffusion is uniform (Gaussian), accounts for the non-Gaussian diffusion often found in complex tissues (23). NODDI offers enhanced precision in brain analysis by focusing on the density and orientation of neurites (24). DTI and NODDI have been used to identify hippocampal microstructural abnormalities predictive of mesial temporal sclerosis (MTS) and to help enhance the characterization of FCD lesions, specifically FCD type IIa and type IIb (25-27). Nevertheless, while these techniques show promise, they have not yet been adopted into clinical practice. Challenges persist regarding their reproducibility, and prospective studies directly comparing these methods against expert clinical radiological evaluations are yet to be conducted.
Double inversion recovery (DIR) is an MRI pulse sequence that suppresses signals from both WM and cerebrospinal fluid (CSF). DIR is particularly sensitive to lesions with low T2 contrast, making it a valuable tool in neuroimaging, especially in the context of epilepsy. It has shown sensitivity between 50% and 88% and specificity between 67% and 91% in detecting FCDs (28). A quantitative analysis revealed a higher signal increase compared to FLAIR in temporal lobe epilepsy (TLE), suggesting DIR may be more sensitive for detecting WM changes such as anterior temporal lobe WM abnormal signal (29). DIR has also been coupled with machine-learning algorithms for its utility in accurately lateralizing and classifying MRI negative TLE (30). DIR also provides high contrast between lesions (e.g., tumours) and adjacent normal-appearing cortex, thus aiding in the delineation of the extent of abnormal tissue prior to surgery (31).
MP2RAGE (Figures 1,2), a magnetization prepared 2 rapid gradient echo sequence, is a modified MPRAGE sequence (32). It is particularly suited for 3 Tesla (T) or higher field scanners and it has been favourably compared to MPRAGE for its improved detection of FCD (33,34). Notably, a recent study proposed an automated MRI post-processing and presentation of co-registered output maps which allowed for a rapid (“within a minute”) identification of FCDs (35).


The 3D edge-enhancing gradient echo (EDGE) technique is an adaptation of the MPRAGE or MP2RAGE sequences, presenting an evident signal void at the healthy GM/WM tissue boundary. It achieves a significantly higher contrast ratio for the subcortical region and the ‘transmantle sign’ (typical area of abnormal neurons between the dysplastic cortex and the ventricle), cited at 95–98%, compared to 17% for MP2RAGE, 19% for FLAIR, and 38% for DIR (36). The use of the EDGE sequence in clinical settings is on the rise (37), with emerging research focused on exploring its feasibility for use with 7-T MRI systems (38).
The fluid and white matter suppression (FLAWS) technique adapts the two inversion times of MP2RAGE to null WM and CSF, respectively, subsequently combining them to emphasise GM. While similar to DIR, FLAWS differs by generating a synthetic image (and, therefore, contrasts) from the acquired images, potentially offering enhanced lesion differentiation. A notable consideration, however, is the acquisition time (ca. 11 minutes) (39). Urushibata et al. (40) showed that FLAWS can provide more homogenous WM signal suppression and better GM visualisation at high magnetic field strengths such as 7 T, demonstrating a better and more uniform WM suppression and a more homogeneous GM delineation compared to DIR. Sun et al. (41) emphasises the promising value of FLAWS in identifying subtle epileptogenic lesions in cases of “MRI-negative” epilepsy, making it a valuable complementary tool to existing morphometric analysis programs (MAP).
Arterial spin labelling (ASL) is a non-invasive MRI sequence measuring cerebral blood flow (CBF), utilising magnetically labelled inflowing blood (Figure 3). It has emerged as a useful tool in the field of neuroimaging, particularly for assessing cerebral perfusion alterations associated with various neurological disorders, including epilepsy (42). ASL can be useful in identifying the epileptogenic focus, both during peri- and interictal periods. Several studies (43-45) have demonstrated the utility of ASL in localising seizures and for presurgical evaluation in NL epilepsy. Additionally, Kojan et al. showed its potential to provide similar predictive power to FDG-PET, with the added advantages of no radiation exposure, greater accessibility, and being less expensive (43). ASL is useful in detecting interictal CBF abnormalities in children with FCD, as illustrated by the work of Blauwblomme et al. (46). Tortora et al. (47) reported that quantitative analyses of ASL maps increase the detection rate of epileptogenic zones in MRI-negative patients compared to a standard qualitative method. Rutten et al. (48) utilised ASL to study the correlation between perfusion of tubers and associated EEG slow waves in children with tuberous sclerosis complex (TSC). Their findings demonstrated that perfusion was reduced in tubers associated with EEG slow waves compared to other tubers. Gennari et al. (49) confirmed that most FCD induce perfusion changes detectable with ASL imaging. They observed that hyperperfused FCDs are associated with a higher spike rate on EEG, indicating a greater degree of epileptogenicity.
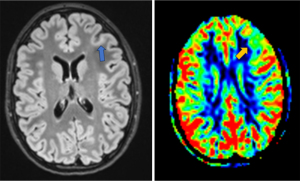
The application of ASL in children comes with challenges, including the lack of standardised methods for acquiring and processing images that can lead to inconsistencies in study results and interpretations. Age-related changes in CBF add complexity to the use of ASL. CBF is initially low in newborns, increases rapidly in the first 6 months of life, and then grows more slowly, peaking between ages 5 and 10 years. The use of sedatives like propofol further complicates CBF measurement, as propofol can indirectly lower CBF by reducing the brain’s oxygen metabolic rate and might directly inhibit vasodilation by affecting vascular nitric oxide signalling. These factors, combined with intrinsic limitations such as the technique’s sensitivity to delayed blood flow, pose significant challenges (50,51). New techniques are being explored, such as multi-delay ASL, which acquires data at multiple time points after the labeling pulse. This method improves the accuracy of CBF measurements by accounting for variations in arterial transit times (52).
In contrast to conventional qualitative MRI sequences that generate ‘weighted images’, quantitative MRI adopts a more analytical approach. It quantifies specific parameters within tissues, providing valuable numerical data that can be used for a more objective evaluation and comparison of tissue properties (53). Several quantitative MRI techniques have been proposed (54), including T1 and T2 relaxation time mapping, the previously mentioned DTI and ASL, quantitative susceptibility mapping (QSM) (55) and MRS.
Quantitative MRI has been utilised to assess changes in cortical architecture in children with heterogeneous drug-resistant focal epilepsy and to investigate whether these changes correlate with disease severity. The study suggests the presence of a potential imaging endophenotype of focal epilepsy that can be detected regardless of radiologically identified abnormalities (56).
QSM is an advanced MRI technique evolving from susceptibility-weighted imaging (SWI). QSM encompasses a set of experimental methods that allow for the precise quantification of absolute concentrations of iron, calcium, and other substances within tissues, based on variations in local magnetic susceptibility. This technique shows potential in identifying epileptogenic tubers in TSC, offering a valuable tool for the detailed assessment of mineral deposits and tissue composition in this condition (57).
MRS is a non-invasive imaging technique that allows the measurement of biochemical metabolites within tissues. Key metabolites that can be measured using MRS include:
- N-acetyl aspartate (NAA): often considered a marker of neuronal health and density.
- Choline (Cho): linked to cell membrane turnover and often elevated in tumors and demyelination.
- Creatine (Cr): reflects cellular energy metabolism.
- Lactate: indicates anaerobic metabolism, often found in conditions of hypoxia or mitochondrial dysfunction.
- Myo-Inositol: associated with glial cell activity and osmotic balance.
- Glutamate and glutamine (Glx): involved in neurotransmission and can be altered in various neurological conditions.
The MRS spectra represent the different frequencies at which these metabolites resonate, producing a graph with peaks corresponding to each metabolite’s concentration. These spectra can aid in differentiating tumors and serve as biomarkers for patient management (58).
Magnetic resonance fingerprinting (MRF) has emerged as a reliable quantitative technique due to its simultaneous measurements of T1 and T2 relaxometry maps, and its more robust performance against measurement inaccuracies in the presence of noise or other acquisition errors (59). MRF has been applied to a variety of neurological and non-neurological diseases (60). In the context of FCDs, numerous studies have investigated the potential of MRF in detecting these abnormalities (61-64). MRF has not yet been integrated into standard clinical practice, and its relatively lengthy acquisition time, approximately 13.5 minutes (59), should be taken into consideration.
Magnetization transfer imaging (MTI) is another technique that measures the exchange of magnetization between free protons in water and protons bound to macromolecules like myelin or membrane lipids. Primarily utilised in multiple sclerosis, MTI has also shown good sensitivity in identifying MCD and acquired cerebral lesions (65). Magnetization transfer T1-weighted imaging can be superior to FLAIR imaging for detecting subtle tubers in TSC (66).
Ultra-high field (UHF) MRI, defined as magnetic fields of 7 T or higher, is increasingly being integrated into clinical practice, offering significant enhancements in both signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR). The increase in SNR with magnetic field strength is noteworthy, with improvements observed across the entire cerebrum: a factor of 3.10±0.20 increase from 3 to 7 T, and a further increase by a factor of 1.76±0.13 from 7 to 9.4 T (67). Furthermore, the CNR was found to be increased by up to 142%, highlighting the substantial benefits of UHF MRI in providing more detailed and accurate imaging (68). These enhancements are critical for improving the visualisation of cortical malformations. Specifically, sequences based on T1-weighted images, like EDGE and MP2RAGE, are optimally suitable for 7-T MRI, providing exceptional detail and clarity in imaging, allowing for better characterization of findings compared to 3 T (69). A 3-T MRI report described “cortical thickness”, whereas a 7-T MRI was able to identify this as PMG and reveal areas of PMG not clearly visible with fields less than 7 T (70). Zucca et al. (71) studied correlations between ex vivo high-resolution imaging and specific histological and ultrastructural patterns in type II FCD to explain the differences in MRI detection of dysplasia and to contribute to the presurgical imaging evaluation of this pathology. The increased use and availability of UHF MRI has led to the development of various protocols, such as the 7-T Epilepsy Task Force’s consensus protocol (72). Additionally, an emerging protocol, referenced as EpiUltraStudy (73), proposes further applications and methodologies utilising 7-T capabilities. UHF MRI is also advantageous for post-processing such as MAP, substantially enhancing the detection rate of FCD (74).
A meta-analysis highlighting the comparison between 7- and 3-T MRI demonstrates the superior ability of 7-T MRI to detect morphological abnormalities, indicating its enhanced diagnostic capabilities. However, as reported, the validity of these findings is limited by the small patient cohorts and lack of standardised evaluation metrics in the existing literature, which necessitates further validation through larger, prospective studies (75). While 7-T MRI has begun clinical application, the use of 9.4-T MRI remains primarily experimental, focusing on advancing imaging resolution and diagnostic accuracy in research settings (76-78).
The transition to UHF MRI is not without its hurdles. The exquisite sensitivity of certain sequences to optimal inversion times and the extreme nonuniformities in the transmit field (B1+) at 7 T make achieving consistent, high-quality imaging across different patients challenging (79). Future research should also clarify whether these imaging advancements at 7 T translate into better surgical outcomes and seizure management postoperatively (79).
Post-processing techniques
Voxel-based morphometry (VBM) was introduced at the end of the 1990s (80) and one of the earliest applications in epilepsy was for TLE in 2002 (81). Different software has been developed for VBM processing, the most common being the statistical parametric mapping (SPM) software and the FMRIB software library (FSL). VBM have been applied firstly and commonly to T1-weighted sequences, later have been also applied to T2-weighted, FLAIR and DIR (82). VBM has been used for the automatic detection of known epileptogenic lesions and in cryptogenic epilepsy, especially in the pre-surgical evaluation (83). Additionally, VBM has been applied in the classification of paediatric TLE with hippocampal sclerosis (84).
One of the most used VBM techniques is MAP (85), which consists of a comparison of the anatomy of the patient’s brain versus a standardised brain derived from healthy controls by analysing differences voxel-to-voxel. At the end of the post-processing, three z-score maps are obtained: extension, junction and thickness which correspond to three of the most typical radiological findings of FCD, such as abnormal sulcal or gyral pattern, blurring of the GM-WM, and abnormal cortical thickness. MAP is intended as a guide in the evaluation of an apparently normal MRI and as a quantitative tool for a more confident diagnosis. In a study investigating its utility in a clinical setting, MAP showed a sensitivity of 0.57, specificity of 0.8, a positive predictive value (PPV) of 0.91, and a negative predictive value (NPV) of 0.35 (86). The authors suggest that the main usefulness of a MAP analysis was to better define the potential epileptogenic zone to guide sEEG implantation to reduce the number of electrodes necessary or even to perform sEEG at all. The main limitations were the lack of established statistical thresholds (z-score maps are evaluated visually) and the added work time (at least 1 hour) for the post-processing and evaluation. Other studies confirmed the usefulness of MAP in focal DRE patients (87,88). Another limitation for its utility in pre-surgical evaluation is that the majority of VBM studies are retrospective and often based on selected cohorts. Thus, false positives remain a very relevant, unsolved issue that all users of post-processing techniques need to be aware of. It requires expertise in interpreting the results and multidisciplinary consensus to reduce the frequency of false-positive and false-negative findings which inevitably occur (82).
Surface-based morphometry (SBM) employs methods similar to VBM, with the primary aim to identify brain lesions within the intricate gyrification that might be missed by VBM. Although SBM (often implemented using FreeSurfer) is a popular tool in studying disorders like autism and schizophrenia, its application in epilepsy research is relatively limited. Researchers like Blackmon et al. (89) have investigated the correlation between cortical boundary blurring and cognitive function in FCD patients. The integration of SBM with machine learning techniques has seen significant developments. Ganji et al. (90) implemented an artificial neural networks (ANN) Classifier on FLAIR sequences, showcasing its diagnostic accuracy in FCD type II for MRI-positive patients. Similarly, Ahmed and colleagues (91) developed an approach that successfully identified FCD in a significant portion (58%) of MRI-negative patients. These studies, while promising, acknowledge the limitations of their dataset and highlight the need for further research with more comprehensive data to substantiate their findings.
fMRI (Figure 4) is a non-invasive imaging technology that measures brain activity by detecting changes associated with blood flow, a technique which is blood oxygenation-level dependent (BOLD). fMRI has been widely used in FCD research, serving multiple purposes. Its applications range from studying functional brain network connectivity alterations and developing algorithms for seizure onset localization to exploring the potential ability to predict the prognosis of surgery (92,93). fMRI can be used in the paediatric population, but there are challenges in patient compliance, whether in performing specific tasks or simply in undergoing the procedure (94). These facets underscore the need for standardised ethical guidelines in paediatric applications, nevertheless the use of fMRI is critical in visualising eloquent areas of the brain (for instance, language lateralisation) in pre-operative planning.

AI
The integration of AI into radiology has led to the development of predictive models that aim to enhance diagnostic accuracy, improve efficiency, and reduce workload for radiologists (95).
As reported in literature (96), several techniques have been developed for FCD detection, categorizable as visual, semi-automatic, and fully automatic, the latter relying on machine learning and deep learning. Naïve Bayes classifiers and support vector machines are the most employed algorithms in this domain. The authors note that the integration of automated tools into FCD detection and localization holds great promise for improving diagnostic accuracy and efficiency. However, these tools suffer from a lack of large datasets, hindering their ability to generalise methods due to insufficient data. There is also an absence of a benchmark dataset for comparing different FCD detection approaches, and no consensus on a single set of measures to validate the results for performance metrics (97).
Deep learning, a subset of AI, employs multi-layered neural networks to analyse complex data, enhancing pattern recognition and decision-making in various fields. Deep learning in neuroimaging for epilepsy shows promise in lesion detection, surgical planning, and outcome prediction, with structural MRI-based studies demonstrating sensitivity up to 90%. Deep learning has been used for the automatic detection of potential PMG in paediatric brain MRI (98). Sollee et al. have broadened the scope of research in epilepsy by incorporating machine learning techniques with fMRI, and extending it to include other advanced methods, like MEG and PET (97). Walger et al. discuss the challenges of applying AI, specifically deep learning, in the detection of FCD in clinical practice. It highlights the difficulties in data collection and ground truth establishment, the variability in neuroimaging modalities and features, and the need for robust validation and external validation for AI models (99). Challenges include the absence of ground-truth labelling and the risk of false positives. The “black box” nature of AI, where the system provides conclusions or decisions without explanations of how they were reached, along with variability in methodologies, affects its clinical acceptance.
The challenge posed by the scarcity of extensive datasets is increasingly being addressed through collaborative efforts by international research centres. These institutions are pooling their data resources in landmark projects such as the Multi-centre Epilepsy Lesion Detection (MELD) and the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) studies. The MELD project (https://github.com/MELDProject) aims to enhance FCD detection using MRI data (Figure 5). The MELD classifier, a machine learning algorithm, utilises surface-based features from magnetic resonance images to identify FCDs (Figure 5). It demonstrated promising sensitivity (59% for the withheld test cohort, 67% including a border zone around lesions) and specificity (54% in the test cohort). This robust and interpretable machine-learning algorithm for automated detection of FCD aims to give physicians greater confidence in the identification of subtle MRI lesions in individuals with epilepsy (100). The MELD project also created an atlas of FCD lesions, enabling the linkage of a patient’s lesion location to presurgical clinical information and further contributing to the understanding of this disease (101).

ENIGMA (https://enigma.ini.usc.edu/ongoing/enigma-epilepsy/), a comprehensive quantitative brain imaging consortium, is committed to exploring neuroimaging abnormality patterns in prevalent epilepsy syndromes such as TLE, extratemporal epilepsy, and genetic generalised epilepsy (102). In one of the ENIGMA studies, Gleichgerrcht et al. (103) assessed the efficacy of machine learning models, particularly support vector machines and deep learning, in differentiating patients with TLE from healthy controls and in lateralizing TLE, using structural and diffusion MRI data.
According to an international survey, there is a generally positive outlook on the use of AI in this field. Healthcare professionals also do not feel that their jobs are at risk due to the integration of AI technologies (104).
Despite the availability of several AI software products in radiology with the CE (Conformité Européenne) mark, the sector remains in its early stages. Of these products, 64% lack peer-reviewed evidence supporting their efficacy. Furthermore, only 18% of these AI products have shown, or have the potential to show, clinical impact. This highlights the need for more rigorous validation and assessment of AI tools in radiology to ensure their effectiveness and clinical relevance (105).
Conclusions
The landscape of neuroimaging is evolving at a rapid pace, and the diagnosis of focal lesions has witnessed substantial advancements. While a range of techniques have been implemented in clinical settings, many remain exclusive to the realm of research and prospective studies are lacking. Acknowledging the constraints of the methodologies discussed is crucial, encompassing the significant time investment required for data acquisition, limitations in data sets and the prevalence of false positives in post-processing techniques, and the lack of consensus on uniform measures for validating AI results alongside peer-reviewed evidence substantiating the effectiveness of AI software products.
Despite these challenges, the future is promising, with a need for more extensive prospective studies and strategies to overcome these obstacles. Collaborative efforts by international research centers, such as the MELD project and the ENIGMA study, are crucial. Future work should focus on model robustness and interpretability. The integration of new MRI sequences and AI in clinical settings will benefit from a multi-specialist approach involving paediatricians, neuroradiologists, and neurosurgeons, enhancing diagnostic and management outcomes and marking a significant step forward in epilepsy care.
Limitations
This narrative review, while comprehensive, carries inherent limitations typical of this type of work. First, the selection of studies included in this review was guided by the authors’ judgment and expertise, which introduces a level of subjectivity. While efforts were made to cover a broad spectrum of relevant literature, the absence of a systematic search strategy may have resulted in the exclusion of pertinent studies, potentially limiting the scope of the analysis.
Additionally, given the dynamic nature of this field, some of the discussions and conclusions may also become less relevant as new data emerge. Readers are encouraged to consider these factors when applying the insights from this review to clinical practice or further research.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-166/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-166/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-166/coif). F.D.A. serves as an unpaid editorial board member of Translational Pediatrics from March 2024 to February 2026. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Epilepsy [Internet]. [cited 2024 Jan 14]. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy
- Global, regional, and national burden of epilepsy, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:357-75. [Crossref] [PubMed]
- Aaberg KM, Gunnes N, Bakken IJ, et al. Incidence and Prevalence of Childhood Epilepsy: A Nationwide Cohort Study. Pediatrics 2017;139:e20163908. [Crossref] [PubMed]
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069-77. [Crossref] [PubMed]
- Dwivedi R, Ramanujam B, Chandra PS, et al. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med 2017;377:1639-47. [Crossref] [PubMed]
- Wang ZI, Alexopoulos AV, Jones SE, et al. The pathology of magnetic-resonance-imaging-negative epilepsy. Mod Pathol 2013;26:1051-8. [Crossref] [PubMed]
- Harvey AS, Cross JH, Shinnar S, et al. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146-55. [Crossref] [PubMed]
- Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, et al. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 2010;89:310-8. [Crossref] [PubMed]
- Eltze CM, Chong WK, Bhate S, et al. Taylor-type focal cortical dysplasia in infants: some MRI lesions almost disappear with maturation of myelination. Epilepsia 2005;46:1988-92. [Crossref] [PubMed]
- Takanashi J, Barkovich AJ. The changing MR imaging appearance of polymicrogyria: a consequence of myelination. AJNR Am J Neuroradiol 2003;24:788-93. [PubMed]
- Yoshida F, Morioka T, Hashiguchi K, et al. Appearance of focal cortical dysplasia on serial MRI after maturation of myelination. Childs Nerv Syst 2008;24:269-73. [Crossref] [PubMed]
- Desikan RS, Barkovich AJ. Malformations of cortical development. Ann Neurol 2016;80:797-810. [Crossref] [PubMed]
- Fauser S, Essang C, Altenmüller DM, et al. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia 2015;56:66-76. [Crossref] [PubMed]
- Wehner T, Weckesser P, Schulz S, et al. Factors influencing the detection of treatable epileptogenic lesions on MRI. A randomized prospective study. Neurol Res Pract 2021;3:41. [Crossref] [PubMed]
- Von Oertzen J, Urbach H, Jungbluth S, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry 2002;73:643-7. [Crossref] [PubMed]
- Juhász C, John F. Utility of MRI, PET, and ictal SPECT in presurgical evaluation of non-lesional pediatric epilepsy. Seizure 2020;77:15-28. [Crossref] [PubMed]
- Sinha N, Johnson GW, Davis KA, et al. Integrating Network Neuroscience Into Epilepsy Care: Progress, Barriers, and Next Steps. Epilepsy Curr 2022;22:272-8. [Crossref] [PubMed]
- Pearce K, Dixon L, D'Arco F, et al. Epilepsy surgery in children: what the radiologist needs to know. Neuroradiology 2020;62:1061-78. [Crossref] [PubMed]
- Jayakar P, Gaillard WD, Tripathi M, et al. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia 2014;55:507-18. [Crossref] [PubMed]
- Huang TH, Lai MC, Chen YS, et al. Brain Imaging in Epilepsy-Focus on Diffusion-Weighted Imaging. Diagnostics (Basel) 2022;12:2602. [Crossref] [PubMed]
- Gennari AG, Cserpan D, Stefanos-Yakoub I, et al. Diffusion tensor imaging discriminates focal cortical dysplasia from normal brain parenchyma and differentiates between focal cortical dysplasia types. Insights Imaging 2023;14:36. [Crossref] [PubMed]
- Arrigoni F, Peruzzo D, Mandelstam S, et al. Characterizing White Matter Tract Organization in Polymicrogyria and Lissencephaly: A Multifiber Diffusion MRI Modeling and Tractography Study. AJNR Am J Neuroradiol 2020;41:1495-502. [Crossref] [PubMed]
- Steven AJ, Zhuo J, Melhem ER. Diffusion kurtosis imaging: an emerging technique for evaluating the microstructural environment of the brain. AJR Am J Roentgenol 2014;202:W26-33. [Crossref] [PubMed]
- Zhang H, Schneider T, Wheeler-Kingshott CA, et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012;61:1000-16. [Crossref] [PubMed]
- Chau Loo Kung G, Chiu A, Davey Z, et al. High-resolution hippocampal diffusion tensor imaging of mesial temporal sclerosis in refractory epilepsy. Epilepsia 2022;63:2301-11. [Crossref] [PubMed]
- Lorio S, Adler S, Gunny R, et al. MRI profiling of focal cortical dysplasia using multi-compartment diffusion models. Epilepsia 2020;61:433-44. [Crossref] [PubMed]
- Gao Y, Zhang Y, Wong CS, et al. Diffusion abnormalities in temporal lobes of children with temporal lobe epilepsy: a preliminary diffusional kurtosis imaging study and comparison with diffusion tensor imaging. NMR Biomed 2012;25:1369-77. [Crossref] [PubMed]
- Wong-Kisiel LC, Britton JW, Witte RJ, et al. Double Inversion Recovery Magnetic Resonance Imaging in Identifying Focal Cortical Dysplasia. Pediatr Neurol 2016;61:87-93. [Crossref] [PubMed]
- Sone D, Sato N, Kimura Y, et al. Quantitative analysis of double inversion recovery and FLAIR signals in temporal lobe epilepsy. Epilepsy Res 2021;170:106540. [Crossref] [PubMed]
- Beheshti I, Sone D, Maikusa N, et al. Accurate lateralization and classification of MRI-negative 18F-FDG-PET-positive temporal lobe epilepsy using double inversion recovery and machine-learning. Comput Biol Med 2021;137:104805. [Crossref] [PubMed]
- Soares BP, Porter SG, Saindane AM, et al. Utility of double inversion recovery MRI in paediatric epilepsy. Br J Radiol 2016;89:20150325. [Crossref] [PubMed]
- Marques JP, Kober T, Krueger G, et al. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 2010;49:1271-81. [Crossref] [PubMed]
- Demerath T, Rubensdörfer L, Schwarzwald R, et al. Morphometric MRI Analysis: Improved Detection of Focal Cortical Dysplasia Using the MP2RAGE Sequence. AJNR Am J Neuroradiol 2020;41:1009-14. [Crossref] [PubMed]
- Demerath T, Kaller CP, Heers M, et al. Fully automated detection of focal cortical dysplasia: Comparison of MPRAGE and MP2RAGE sequences. Epilepsia 2022;63:75-85. [Crossref] [PubMed]
- Urbach H, Heers M, Altenmueller DM, et al. “Within a minute” detection of focal cortical dysplasia. Neuroradiology 2022;64:715-26. [Crossref] [PubMed]
- Middlebrooks EH, Lin C, Westerhold E, et al. Improved detection of focal cortical dysplasia using a novel 3D imaging sequence: Edge-Enhancing Gradient Echo (3D-EDGE) MRI. Neuroimage Clin 2020;28:102449. [Crossref] [PubMed]
- Abula Y, Abulimiti A, Liu Z, et al. The Role of the Three-Dimensional Edge-Enhancing Gradient Echo Sequence at 3T MRI in the Detection of Focal Cortical Dysplasia: A Technical Case Report and Literature Review. Neuropediatrics 2022;53:436-9. [Crossref] [PubMed]
- Tao S, Zhou X, Greco E, et al. Edge-Enhancing Gradient-Echo MP2RAGE for Clinical Epilepsy Imaging at 7T. AJNR Am J Neuroradiol 2023;44:268-70. [Crossref] [PubMed]
- Chen X, Qian T, Kober T, et al. Gray-matter-specific MR imaging improves the detection of epileptogenic zones in focal cortical dysplasia: A new sequence called fluid and white matter suppression (FLAWS). Neuroimage Clin 2018;20:388-97. [Crossref] [PubMed]
- Urushibata Y, Kuribayashi H, Fujimoto K, et al. Advantages of fluid and white matter suppression (FLAWS) with MP2RAGE compared with double inversion recovery turbo spin echo (DIR-TSE) at 7T. Eur J Radiol 2019;116:160-4. [Crossref] [PubMed]
- Sun K, Yu T, Yang D, et al. Fluid and White Matter Suppression Imaging and Voxel-Based Morphometric Analysis in Conventional Magnetic Resonance Imaging-Negative Epilepsy. Front Neurol 2021;12:651592. [Crossref] [PubMed]
- Lindner T, Bolar DS, Achten E, et al. Current state and guidance on arterial spin labeling perfusion MRI in clinical neuroimaging. Magn Reson Med 2023;89:2024-47. [Crossref] [PubMed]
- Kojan M, Gajdoš M, Říha P, et al. Arterial Spin Labeling is a Useful MRI Method for Presurgical Evaluation in MRI-Negative Focal Epilepsy. Brain Topogr 2021;34:504-10. [Crossref] [PubMed]
- Zhang J, Zhang H, Li Y, et al. Arterial spin labeling for presurgical localization of refractory frontal lobe epilepsy in children. Eur J Med Res 2021;26:88. [Crossref] [PubMed]
- Lee SM, Kwon S, Lee YJ. Diagnostic usefulness of arterial spin labeling in MR negative children with new onset seizures. Seizure 2019;65:151-8. [Crossref] [PubMed]
- Blauwblomme T, Boddaert N, Chémaly N, et al. Arterial Spin Labeling MRI: a step forward in non-invasive delineation of focal cortical dysplasia in children. Epilepsy Res 2014;108:1932-9. [Crossref] [PubMed]
- Tortora D, Cataldi M, Severino M, et al. Comparison of Qualitative and Quantitative Analyses of MR-Arterial Spin Labeling Perfusion Data for the Assessment of Pediatric Patients with Focal Epilepsies. Diagnostics (Basel) 2022;12:811. [Crossref] [PubMed]
- Rutten C, Fillon L, Kuchenbuch M, et al. The longitudinal evolution of cerebral blood flow in children with tuberous sclerosis assessed by arterial spin labeling magnetic resonance imaging may be related to cognitive performance. Eur Radiol 2023;33:196-206. [Crossref] [PubMed]
- Gennari AG, Bicciato G, Lo Biundo SP, et al. Lesion volume and spike frequency on EEG impact perfusion values in focal cortical dysplasia: a pediatric arterial spin labeling study. Sci Rep 2024;14:7601. [Crossref] [PubMed]
- Hales PW, d'Arco F, Cooper J, et al. Arterial spin labelling and diffusion-weighted imaging in paediatric brain tumours. Neuroimage Clin 2019;22:101696. [Crossref] [PubMed]
- Proisy M, Bruneau B, Rozel C, et al. Arterial spin labeling in clinical pediatric imaging. Diagn Interv Imaging 2016;97:151-8. [Crossref] [PubMed]
- Chen G, Lei D, Ren J, et al. Patterns of postictal cerebral perfusion in idiopathic generalized epilepsy: a multi-delay multi-parametric arterial spin labelling perfusion MRI study. Sci Rep 2016;6:28867. [Crossref] [PubMed]
- Pierpaoli C. Quantitative brain MRI. Top Magn Reson Imaging 2010;21:63. [Crossref] [PubMed]
- Granziera C, Wuerfel J, Barkhof F, et al. Quantitative magnetic resonance imaging towards clinical application in multiple sclerosis. Brain 2021;144:1296-311. [Crossref] [PubMed]
- Lorio S, Sedlacik J, So PW, et al. Quantitative MRI susceptibility mapping reveals cortical signatures of changes in iron, calcium and zinc in malformations of cortical development in children with drug-resistant epilepsy. Neuroimage 2021;238:118102. [Crossref] [PubMed]
- Casella C, Vecchiato K, Cromb D, et al. Widespread, depth-dependent cortical microstructure alterations in pediatric focal epilepsy. Epilepsia 2024;65:739-52. [Crossref] [PubMed]
- Chari A, Sedlacik J, Seunarine K, et al. Epileptogenic Tubers Are Associated with Increased Kurtosis of Susceptibility Values: A Combined Quantitative Susceptibility Mapping and Stereoelectroencephalography Pilot Study. AJNR Am J Neuroradiol 2023;44:974-82. [Crossref] [PubMed]
- Blüml S, Saunders A, Tamrazi B, Proton MR. Spectroscopy of Pediatric Brain Disorders. Diagnostics (Basel) 2022;12:1462. [Crossref] [PubMed]
- Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495:187-92. [Crossref] [PubMed]
- Panda A, Mehta BB, Coppo S, et al. Magnetic Resonance Fingerprinting-An Overview. Curr Opin Biomed Eng 2017;3:56-66. [Crossref] [PubMed]
- Ma D, Jones SE, Deshmane A, et al. Development of high-resolution 3D MR fingerprinting for detection and characterization of epileptic lesions. J Magn Reson Imaging 2019;49:1333-46. [Crossref] [PubMed]
- Su TY, Tang Y, Choi JY, et al. Evaluating whole-brain tissue-property changes in MRI-negative pharmacoresistant focal epilepsies using MR fingerprinting. Epilepsia 2023;64:430-42. [Crossref] [PubMed]
- Nöth U, Gracien RM, Maiworm M, et al. Detection of cortical malformations using enhanced synthetic contrast images derived from quantitative T1 maps. NMR Biomed 2020;33:e4203. [Crossref] [PubMed]
- Maiworm M, Nöth U, Hattingen E, et al. Improved Visualization of Focal Cortical Dysplasia With Surface-Based Multiparametric Quantitative MRI. Front Neurosci 2020;14:622. [Crossref] [PubMed]
- Rugg-Gunn FJ, Eriksson SH, Boulby PA, et al. Magnetization transfer imaging in focal epilepsy. Neurology 2003;60:1638-45. [Crossref] [PubMed]
- Girard N, Zimmerman RA, Schnur RE, et al. Magnetization transfer in the investigation of patients with tuberous sclerosis. Neuroradiology 1997;39:523-8. [Crossref] [PubMed]
- Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn Reson Med 2016;75:801-9. [Crossref] [PubMed]
- DiFrancesco MW, Rasmussen JM, Yuan W, et al. Comparison of SNR and CNR for in vivo mouse brain imaging at 3 and 7 T using well matched scanner configurations. Med Phys 2008;35:3972-8. [Crossref] [PubMed]
- Middlebrooks EH, Greco E, Zhou X, et al. Edge-Enhancing Gradient Echo MRI at 7T for detection of focal cortical dysplasia in epilepsy. Neuroimage: Reports 2023;3:100187. [Crossref]
- Bartolini E, Cosottini M, Donatelli G, et al. Does 7T MRI reveal a neuronal bridge between periventricular heterotopia and overlying cortical malformations? Seizure 2022;103:99-100. [Crossref] [PubMed]
- Zucca I, Milesi G, Medici V, et al. Type II focal cortical dysplasia: Ex vivo 7T magnetic resonance imaging abnormalities and histopathological comparisons. Ann Neurol 2016;79:42-58. [Crossref] [PubMed]
- Opheim G, van der Kolk A, Markenroth Bloch K, et al. 7T Epilepsy Task Force Consensus Recommendations on the Use of 7T MRI in Clinical Practice. Neurology 2021;96:327-41. [Crossref] [PubMed]
- van Lanen RHGJ, Wiggins CJ, Colon AJ, et al. Value of ultra-high field MRI in patients with suspected focal epilepsy and negative 3 T MRI (EpiUltraStudy): protocol for a prospective, longitudinal therapeutic study. Neuroradiology 2022;64:753-64. [Crossref] [PubMed]
- Wang I, Oh S, Blümcke I, et al. Value of 7T MRI and post-processing in patients with nonlesional 3T MRI undergoing epilepsy presurgical evaluation. Epilepsia 2020;61:2509-20. [Crossref] [PubMed]
- Park JE, Cheong EN, Jung DE, et al. Utility of 7 Tesla Magnetic Resonance Imaging in Patients With Epilepsy: A Systematic Review and Meta-Analysis. Front Neurol 2021;12:621936. [Crossref] [PubMed]
- Yushkevich PA, Avants BB, Pluta J, et al. A high-resolution computational atlas of the human hippocampus from postmortem magnetic resonance imaging at 9.4 T. Neuroimage 2009;44:385-98. [Crossref] [PubMed]
- Reeves C, Tachrount M, Thomas D, et al. Combined Ex Vivo 9.4T MRI and Quantitative Histopathological Study in Normal and Pathological Neocortical Resections in Focal Epilepsy. Brain Pathol 2016;26:319-33. [Crossref] [PubMed]
- Kwan BYM, Salehi F, Kope R, et al. Evaluation of ex-vivo 9.4T MRI in post-surgical specimens from temporal lobe epilepsy patients. J Neuroradiol 2017;44:377-80. [Crossref] [PubMed]
- van Lanen RHGJ, Colon AJ, Wiggins CJ, et al. Ultra-high field magnetic resonance imaging in human epilepsy: A systematic review. Neuroimage Clin 2021;30:102602. [Crossref] [PubMed]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage 2000;11:805-21. [Crossref] [PubMed]
- Keller SS, Mackay CE, Barrick TR, et al. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 2002;16:23-31. [Crossref] [PubMed]
- Martin P, Bender B, Focke NK. Post-processing of structural MRI for individualized diagnostics. Quant Imaging Med Surg 2015;5:188-203. [PubMed]
- Martin P, Winston GP, Bartlett P, et al. Voxel-based magnetic resonance image postprocessing in epilepsy. Epilepsia 2017;58:1653-64. [Crossref] [PubMed]
- Chen S, Zhang J, Ruan X, et al. Voxel-based morphometry analysis and machine learning based classification in pediatric mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Imaging Behav 2020;14:1945-54. [Crossref] [PubMed]
- Huppertz HJ, Grimm C, Fauser S, et al. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res 2005;67:35-50. [Crossref] [PubMed]
- González-Ortiz S, Medrano S, Capellades J, et al. Voxel-based morphometry for the evaluation of patients with pharmacoresistant epilepsy with apparently normal MRI. J Neuroimaging 2021;31:560-8. [Crossref] [PubMed]
- Wong-Kisiel LC, Tovar Quiroga DF, Kenney-Jung DL, et al. Morphometric analysis on T1-weighted MRI complements visual MRI review in focal cortical dysplasia. Epilepsy Res 2018;140:184-91. [Crossref] [PubMed]
- Fearns N, Birk D, Bartkiewicz J, et al. Quantitative analysis of the morphometric analysis program MAP in patients with truly MRI-negative focal epilepsy. Epilepsy Res 2023;192:107133. [Crossref] [PubMed]
- Blackmon K, Kuzniecky R, Barr WB, et al. Cortical Gray-White Matter Blurring and Cognitive Morbidity in Focal Cortical Dysplasia. Cereb Cortex 2015;25:2854-62. [Crossref] [PubMed]
- Ganji Z, Hakak MA, Zamanpour SA, et al. Automatic Detection of Focal Cortical Dysplasia Type II in MRI: Is the Application of Surface-Based Morphometry and Machine Learning Promising? Front Hum Neurosci 2021;15:608285. [Crossref] [PubMed]
- Ahmed B, Brodley CE, Blackmon KE, et al. Cortical feature analysis and machine learning improves detection of "MRI-negative" focal cortical dysplasia. Epilepsy Behav 2015;48:21-8. [Crossref] [PubMed]
- Yao L, Cheng N, Chen AQ, et al. Advances in Neuroimaging and Multiple Post-Processing Techniques for Epileptogenic Zone Detection of Drug-Resistant Epilepsy. J Magn Reson Imaging 2023; Epub ahead of print. [Crossref] [PubMed]
- Songjiang L, Tijiang Z, Heng L, et al. Impact of Brain Functional Network Properties on Intelligence in Children and Adolescents with Focal Epilepsy: A Resting-state MRI Study. Acad Radiol 2021;28:225-32. [Crossref] [PubMed]
- Kraus D, Horowitz-Kraus T. Functional MRI research involving healthy children: Ethics, safety and recommended procedures. Acta Paediatr 2022;111:741-9. [Crossref] [PubMed]
- What the radiologist should know about artificial intelligence - an ESR white paper. Insights Imaging 2019;10:44. [Crossref] [PubMed]
- Jiménez-Murillo D, Castro-Ospina AE, Duque-Muñoz L, et al. Automatic Detection of Focal Cortical Dysplasia Using MRI: A Systematic Review. Sensors (Basel) 2023;23:7072. [Crossref] [PubMed]
- Sollee J, Tang L, Igiraneza AB, et al. Artificial intelligence for medical image analysis in epilepsy. Epilepsy Res 2022;182:106861. [Crossref] [PubMed]
- Zhang L, Abdeen N, Lang J. A novel center-based deep contrastive metric learning method for the detection of polymicrogyria in pediatric brain MRI. Comput Med Imaging Graph 2024;114:102373. [Crossref] [PubMed]
- Walger L, Adler S, Wagstyl K, et al. Artificial intelligence for the detection of focal cortical dysplasia: Challenges in translating algorithms into clinical practice. Epilepsia 2023;64:1093-112. [Crossref] [PubMed]
- Spitzer H, Ripart M, Whitaker K, et al. Interpretable surface-based detection of focal cortical dysplasias: a Multi-centre Epilepsy Lesion Detection study. Brain 2022;145:3859-71. [Crossref] [PubMed]
- Wagstyl K, Whitaker K, Raznahan A, et al. Atlas of lesion locations and postsurgical seizure freedom in focal cortical dysplasia: A MELD study. Epilepsia 2022;63:61-74. [Crossref] [PubMed]
- Hatton SN, Huynh KH, Bonilha L, et al. White matter abnormalities across different epilepsy syndromes in adults: an ENIGMA-Epilepsy study. Brain 2020;143:2454-73. [Crossref] [PubMed]
- Gleichgerrcht E, Munsell BC, Alhusaini S, et al. Artificial intelligence for classification of temporal lobe epilepsy with ROI-level MRI data: A worldwide ENIGMA-Epilepsy study. Neuroimage Clin 2021;31:102765. [Crossref] [PubMed]
- Shelmerdine SC, Rosendahl K, Arthurs OJ. Artificial intelligence in paediatric radiology: international survey of health care professionals’ opinions. Pediatr Radiol 2022;52:30-41. [Crossref] [PubMed]
- van Leeuwen KG, Schalekamp S, Rutten MJCM, et al. Artificial intelligence in radiology: 100 commercially available products and their scientific evidence. Eur Radiol 2021;31:3797-804. [Crossref] [PubMed]


