
Efficacy and safety of spleen aminopeptide oral solution for children with allergic rhinitis and adenoid hypertrophy: a randomised trial
Highlight box
Key findings
• The spleen aminopeptide oral solution (SAOS) could significantly reduce the size of adenoids and improve some clinical symptoms in children with allergic rhinitis (AR) and adenoid hypertrophy (AH) after 8 weeks of treatments.
• No adverse reactions of SAOS were observed during the 8 weeks of treatments.
What is known and what is new?
• SAOS is clinically used as an adjunct therapy for allergic diseases such as AR and asthma, and it is safe and effective.
• In children with AR and AH, SAOS as an adjunct therapy for 8 weeks can significantly reduce the size of the adenoids and alleviate some clinical symptoms of AH. No adverse reactions were observed during the treatment period.
What is the implication, and what should change now?
• For children with AR and AH, SAOS can be provided as an adjunct therapy, which is effective and safe.
Introduction
Allergic rhinitis (AR) is a common allergic disease characterized by an immunoglobulin E (IgE)-mediated inflammatory reaction when the nasal mucosa comes into contact with inhaled allergens. Although AR might seem like a minor issue, symptoms such as nasal congestion, rhinorrhea, and nasal itching can significantly affect the life quality of patients. The incidence of AR is increasing rapidly due to global climate and environmental changes, and it is currently reported to affect approximately 25–40% peoples globally (1).
Adenoids are small lumps of tissue located behind the nose in the upper airway and are part of the immune system. Adenoids are largest when children are around 2–6 years old and gradually regress around 10–12 years old. Adenoid hypertrophy (AH) refers to the enlargement of the adenoids. It is a common condition in pediatric otolaryngology and can lead to nasal obstruction, nighttime snoring, mouth breathing, and even respiratory pauses. In severe cases, AH may also cause maxillofacial developmental disorders (adenoid facies), delayed growth, and neurobehavioral disorders (2).
The etiology of AH is not fully understood, but both allergies and infections are considered risk factors (3-6). AH is closely related to AR. On one hand, AH can block the nasal passages, impair ventilation and drainage of the nasal cavity and sinuses, thereby increasing the inflammatory response of AR. On the other hand, the inflammatory secretions from AR can repeatedly stimulate the adenoids, leading to increased hypertrophy, particularly in cases associated with postnasal drip syndrome. Warman et al. studied the effects of adenoidectomy in children with and without AR, showing that symptoms improved after surgery in both groups; however, children with AR benefited more than those without AR. In other words, treating AH is beneficial for controlling AR (7). It is well known that AR is associated with an IgE-mediated inflammatory reaction, and it has also been reported that AH is an important pathological result of this reaction (8). Cho et al. (9) measured 21 specific IgE antibodies in serum and adenoid tissue from 102 children with AH, finding that 70.6% of these children were sensitive to more than one allergen in serum and/or adenoid tissue, indicating that allergic reactions may be a risk factor for AH. In summary, AH is closely related to AR, and the two conditions mutually influence each other.
The treatment of AH includes both surgical and non-surgical approaches. Given the anesthesia risks and potential complications associated with surgery, many patients prefer non-surgical treatments, including medications and adjunctive therapies (10-15). Commonly used medications include leukotriene receptor antagonists (such as montelukast), antibiotics (for concurrent sinusitis or upper respiratory tract infections), and intranasal corticosteroids. Adjunctive therapies include nasal irrigation, herbal treatments, bacteriotherapy, and halotherapy. This study explored a novel therapy, spleen aminopeptide oral solution (SAOS), for AH. SAOS is a solution containing a mixture of polypeptide amino acids and polynucleotides derived from healthy bovine spleen. In China, SAOS is widely used for treating AR, hepatitis, and other diseases, and it has been proven to be effective and safe. It is an emerging treatment option that focuses on modulating the immune system to address the underlying causes of allergic reactions, including immune modulation, anti-inflammatory effects, and mucosal immunity enhancement (16,17). However, the efficacy and safety of SAOS for treating children with AR combined with AH are still unclear.
This study established a randomized, controlled clinical trial to treat children with AR and AH for two months. The control group received loratadine (1 month) and isotonic saline solution (2 months), while the SAOS group received loratadine (1 month), isotonic saline solution (2 months), and SAOS (2 months). The study aimed to assess the improvement in adenoid size and clinical symptoms in children with AR and AH. We present this article in accordance with the CONSORT reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-203/rc).
Methods
Study design and patients
This was a prospective, randomized, controlled study. Children with AR and AH who visited the Otolaryngology Department of the Children’s Hospital Affiliated with Fudan University between June 2022 and April 2023 were included. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Children’s Hospital Affiliated with Fudan University (No. 2021-423-04), and informed consent was obtained from the patients’ parents or legal guardians. The study was registered on one of the World Health Organization (WHO)-recognized clinical trial registry websites (https://www.chictr.org.cn/) (registration number: ChiCTR2200056763). The flowchart of the study design is shown in Figure 1.

Inclusion criteria were as follows: (I) patients aged between 3 and 12 years; (II) patients diagnosed with AR and confirmed to be allergic to dust mites or pollen; (III) patients diagnosed with AH, showing grade II or higher obstruction of the nostrils by nasal endoscopy, and an adenoid-to-nasopharynx (A/N) ratio between 0.61 and 0.90 on a lateral X-ray; (IV) patients with a history of nasal congestion, coughing, and snoring for more than 3 months; (V) patients who had not received any previous treatment with other immunomodulatory drugs within the past 4 weeks; (VI) patients without systemic corticosteroid treatment within the past 3 months; (VII) patients willing to participate in the study and who signed an informed consent form.
Exclusion criteria were as follows: (I) patients who had undergone adenoidectomy; (II) patients with nasal-sinusitis related to grade II or higher tonsil hypertrophy, including nasal polyps, severe deviated nasal septum, glossopharyngeal muscle tension, nasal cavity masses, retroverted tongue base, and obstruction symptoms caused by obesity; (III) patients with acute upper or lower respiratory tract infection within the past 2 weeks; (IV) patients with metabolic or immunodeficiency diseases, as well as severe organic diseases affecting the heart, liver, kidneys, brain, etc.; (V) patients deemed unsuitable for clinical research by the researchers.
Termination/dropout criteria for the patients were as follows: (I) occurrence of severe adverse events or other significant physiological changes that prevent the continuation of the study; (II) non-compliance with the treatment protocol; and (III) voluntary withdrawal from the study.
Grouping and treatments
Patients were randomly assigned to either the control group (n=50) or the SAOS group (n=50) using a random number table.
The treatments for the control group included oral administration of Loratadine Syrup for 1 month and isotonic saline nasal spray for 2 months. The treatments for the SAOS group included oral administration of Loratadine Syrup for 1 month, isotonic saline nasal spray for 2 months, and oral administration of SAOS for 2 months.
Loratadine Syrup (Bayer, Germany), the first-line medication for the treatment of AR, was administered at a concentration of 0.1% (60 mL: 60 mg) before bedtime, with 5 mL/day for individuals weighing less than 30 kg and 10 mL/day for individuals weighing more than 30 kg. Isotonic saline nasal spray was administered three times a day, with approximately 5 mL per spray. SAOS (10 mL, Beijing No. 1 Biochemical Pharmaceutical Co., Ltd., China) consisted of 10 mg peptides (calculated as bovine serum albumin) and 0.1 mg nucleotides (calculated as D-ribose). For children aged 1–3 years, SAOS was administered at 10 mL per dose and once every other day. For children aged 4–5 years, SAOS was administered at 10 mL per dose and once daily for the first 5 days, then once every other day. For children aged 6–14 years, SAOS was administered at 10 mL per dose daily.
Outcomes
The primary outcome was the A/N ratio, and the secondary outcomes included nasal symptom score, AH score, and medication score. The evaluation of the primary outcome was carried out before treatment (T0), after 1 month of treatment (T1), and after 2 months of treatment (T2).
- A/N ratio: an A/N ratio of ≤0.70 indicated mild hypertrophy, a ratio between 0.71 and 0.80 indicated moderate hypertrophy, and a ratio >0.80 indicated severe hypertrophy. Detailed information on the measurement is provided in Supplementary file (Appendix 1).
- Nasal symptom score: a visual analog scale (VAS, 10 cm long) was used, with “no discomfort” [0] on the left and “severe discomfort” [10] on the right. Family members marked the perceived level of discomfort based on the clinical symptoms of the child, and the score was determined accordingly. The scoring method is described in Supplementary file (Appendix 2).
- AH score: the symptoms of nasal congestion, snoring, mouth breathing, and restless sleep before and after treatment were observed and recorded by family members using a scale ranging from 0 to 4 points. The scoring method is detailed in Supplementary file (Appendix 3).
- Medication score: the concurrent use of medications by patients was not restricted in this study. For each day, the use of oral and/or topical antihistamines (nasal or ocular), nasal corticosteroid, and oral corticosteroid were scored as 1, 2 and 3 points, respectively. The β2-agonists administered for asthma were scored as 1 point per day, and inhaled corticosteroids were scored as 2 points per day. The total medication score was the cumulative score of all these drugs. The specific scoring scheme is described in Supplementary file (Appendix 4).
Safety evaluation
Blood and urine samples were collected from the patients at T0 and T2 for safety evaluation. The blood routine tests included platelet (Plt) count, white blood cell (WBC) count, lymphocyte (LY) count, granulocyte percentage (GR%), red blood cell (RBC) count, hemoglobin (Hb), hematocrit, and eosinophil (EOS) count. The urine routine tests included WBC count, RBC count, protein, glucose, occult blood (OB) test, ketones, bilirubin, and urobilinogen (URO). The adverse events, including their types, frequency, and relationship to the medication, were recorded. Detailed explanations were provided for any study termination or severe/serious adverse events.
Statistical analysis
The per-protocol set (PPS), which includes all patients who meet the inclusion criteria, was used for the efficacy evaluation, while the safety set (SS) was used for safety evaluation. The last observation carried forward (LOCF) principle was employed to handle missing data in the efficacy evaluation.
Statistical analysis was performed using SPSS 26.0. The normality of the data distribution was examined using the Shapiro-Wilk test. Continuous data with a normal distribution were presented as mean ± standard deviation (SD). Multiple group comparisons were conducted using one-way analysis of variance (ANOVA), with the least significant difference (LSD) and Tamhane methods for post hoc comparisons. Data with a skewed distribution were described using the median (interquartile range, IQR) and compared using the Mann-Whitney U test. Categorical data were presented as frequencies and percentages (%) and compared using the Chi-squared test among groups. Data from repeated measurements were analyzed using repeated-measures ANOVA. A P value of less than 0.05 was considered statistically significant.
Results
Comparison of baseline characteristics between the control group and SAOS group
A total of 100 patients were enrolled in this study, with 50 in the SAOS group and 50 in the control group. However, 22 patients dropped out due to serious protocol violations. Based on the PPS dataset, 78 patients were included, with 36 in the control group and 42 in the SAOS group. Results of ANOVA showed no statistically significant differences between the two groups in baseline age (Z=−0.425, P=0.67), A/N ratio (Z=−0.524, P=0.60), nasal symptom scores [including sneezing (Z=−0.201, P=0.84), runny nose (Z=−0.170, P=0.87), nasal itching (Z=−1.474, P=0.14), nasal congestion (Z=−0.948, P=0.34)], and AH scores [including nasal congestion (Z=−0.497, P=0.62), snoring (Z=−1.767, P=0.08), mouth breathing (Z=−1.515, P=0.13), and restless sleep (Z=−0.141, P=0.89)]. However, the distribution of gender was significantly different between the groups (χ2=6.404, P=0.01) (Table 1).
Table 1
| Indices | Control group (n=36) | SAOS group (n=42) | χ2/Z | P |
|---|---|---|---|---|
| Gender | 6.404† | 0.01 | ||
| Male | 28 | 21 | ||
| Female | 8 | 21 | ||
| Age (years) | 4.00 (3.25, 6.00) | 5.00 (4.00, 6.00) | −0.425‡ | 0.67 |
| A/N ratio | 0.72 (0.69, 0.77) | 0.73 (0.70, 0.78) | −0.524‡ | 0.60 |
| Nasal symptom score | ||||
| Sneezing | 3.00 (2.25, 4.00) | 3.00 (2.00, 4.00) | −0.201‡ | 0.84 |
| Runny nose | 3.00 (1.00, 3.00) | 3.00 (0.75, 5.00) | −0.170‡ | 0.87 |
| Nasal itching | 4.00 (3.00, 6.00) | 5.00 (3.00, 6.00) | −1.474‡ | 0.14 |
| Nasal congestion | 6.00 (4.00, 7.75) | 5.00 (4.00, 7.00) | −0.948‡ | 0.34 |
| Adenoid hypertrophy score | ||||
| Nasal congestion | 2.00 (1.25, 3.00) | 2.00 (1.75, 3.00) | −0.497‡ | 0.62 |
| Snoring | 2.50 (2.00, 3.00) | 2.00 (1.00, 3.00) | −1.767‡ | 0.08 |
| Mouth breathing | 2.25 (1.00, 3.00) | 2.00 (1.00, 3.00) | −1.515‡ | 0.13 |
| Restless sleep | 2.00 (1.00, 3.00) | 2.00 (1.00, 2.25) | −0.141‡ | 0.89 |
Data are presented as number or median (interquartile range P25, P75). †, Chi-square test; ‡, Mann-Whitney U test. SAOS, spleen aminopeptide oral solution; A/N, adenoid-to-nasopharynx.
Comparison of A/N ratio and adenoid size between the control group and SAOS group at T0 and T2
As shown in Table 2, there was no statistically significant difference in baseline A/N ratio between the control group and the SAOS group at T0. However, the A/N ratio in the SAOS group was significantly lower than in the control group at T2 (Z=−3.536, P<0.001). Additionally, there was no statistically significant difference in the distribution of adenoid size between the two groups at T0 (χ²=0.314, P=0.86). A significant difference was observed between groups at T2 (χ²=15.875, P=0.001), and the proportion of moderate and severe hypertrophy in the SAOS group (11.90%) was significantly lower than those in the control group (52.78%). These findings suggest that after 8 weeks of treatment, the SAOS group experienced a greater reduction in adenoid size compared to the control group.
Table 2
| Indices | Control group (n=36) | SAOS group (n=42) | χ2/Z | P |
|---|---|---|---|---|
| A/N ratio at T0 | 0.72 (0.69, 0.77) | 0.73(0.70, 0.78) | −0.524‡ | 0.60 |
| Adenoid size at T0 | 0.314† | 0.86 | ||
| Mild hypertrophy | 16 (44.44) | 17 (40.48) | ||
| Moderate hypertrophy | 17 (47.22) | 20 (47.62) | ||
| Severe hypertrophy | 3 (8.33) | 5 (11.9) | ||
| A/N ratio at T2 | 0.72 (0.62, 0.78) | 0.62 (0.55, 0.70) | −3.536‡ | <0.001* |
| Adenoid size at T2 | 15.875† | 0.001* | ||
| Not hypertrophied | 7 (19.44) | 20 (47.62) | ||
| Mild hypertrophy | 10 (27.78) | 17 (40.48) | ||
| Moderate hypertrophy | 15 (41.67) | 4 (9.52) | ||
| Severe hypertrophy | 4 (11.11) | 1 (2.38) |
Data are presented as median (interquartile range P25, P75) or n (%). †, Chi-square test; ‡, Mann-Whitney U test; *, P<0.05 for statistical significance. Mild hypertrophy: 0.61≤ A/N ≤0.70; moderate hypertrophy: 0.71≤ A/N ≤0.80; severe hypertrophy: A/N >0.80. A/N, adenoid-to-nasopharynx; SAOS, spleen aminopeptide oral solution; T0, before treatment; T2, after 2 months of treatment.
Comparison of nasal symptom scores and AH scores between the control group and SAOS group at T0 and T2
As shown in Figure 2 and Table 3, with increasing duration of medication, there was a significant decreasing trend in these scores: sneezing (F=52.806, P<0.001), runny nose (F=28.802, P<0.001), nasal itching (F=101.272, P<0.001), and nasal congestion (F=83.349, P<0.001). These findings suggest that both the control group and the SAOS group experienced improvement in nasal symptoms, with the improvement being more significant over time. Nonetheless, there was no statistical difference between the two groups.
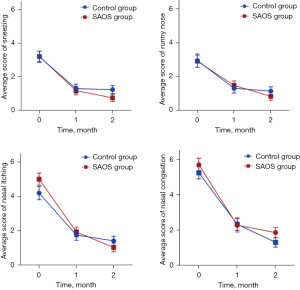
Table 3
| Symptoms | Timepoints | Control group (n=36) | SAOS group (n=42) | F† | P |
|---|---|---|---|---|---|
| Sneezing | T0 | 3.00 (2.25, 4.00) | 3.00 (2.00, 4.00) | 52.806 | <0.001 |
| T1 | 1.00 (0.00, 2.00) | 1.00 (0.00, 1.25) | |||
| T2 | 0.50 (0.00, 2.00) | 1.00 (0.00, 1.00) | |||
| Runny nose | T0 | 3.00 (1.00, 3.00) | 3.00 (0.75, 5.00) | 28.802 | <0.001 |
| T1 | 0.50 (0.00, 2.00) | 1.00 (0.00, 2.00) | |||
| T2 | 0.00 (0.00, 2.00) | 0.00 (0.00, 2.00) | |||
| Nasal itching | T0 | 4.00 (3.00, 6.00) | 5.00 (3.00, 6.00) | 101.272 | <0.001 |
| T1 | 1.00 (0.00, 3.00) | 2.00 (0.00, 3.00) | |||
| T2 | 1.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | |||
| Nasal congestion | T0 | 6.00 (4.00, 7.75) | 5.00 (3.00, 7.00) | 83.349 | < 0.001 |
| T1 | 2.00 (0.25, 3.00) | 2.00 (0.75, 3.25) | |||
| T2 | 1.00 (0.00, 3.00) | 1.00 (0.00, 2.25) |
Data are presented as median (interquartile range P25, P75). †, repeated-measures ANOVA. SAOS, spleen aminopeptide oral solution; T0, before treatment; T1, after 1 month of treatment; T2, after 2 months of treatment; ANOVA, analysis of variance.
As shown in Table 4, there was no difference in AH scores between the two groups at T0 (P>0.05). However, significant improvements were observed in mouth breathing (Z=−2.650, P=0.008) and restless sleep (Z=−2.759, P=0.006) in the SAOS group compared to the control group at T2. In contrast, there were no significant differences in nasal congestion (Z=−1.410, P=0.16) and snoring (Z=−1.407, P=0.16) between the two groups. These findings suggest that the SAOS group showed improvement in some AH symptoms after 8 weeks of treatment compared to the control group, though complete improvement may require longer treatment.
Table 4
| Symptoms | Timepoints | Control group (n=36) | SAOS group (n=42) | Z† | P |
|---|---|---|---|---|---|
| Nasal congestion | T0 | 2.00 (1.25, 3.00) | 2.00 (1.75, 3.00) | −0.497 | 0.62 |
| T1 | 1.00 (1.00, 2.00) | 1.00 (0.50, 2.00) | −0.085 | 0.93 | |
| T2 | 1.00 (0.00, 2.00) | 1.00 (0.00, 1.00) | −1.410 | 0.16 | |
| Snoring | T0 | 2.00 (2.00, 3.00) | 2.00 (1.00, 3.00) | −1.767 | 0.08 |
| T1 | 1.00 (0.00, 2.00) | 1.00 (0.00, 2.00) | −0.723 | 0.47 | |
| T2 | 1.00 (0.00, 1.75) | 0.00 (0.00, 1.00) | −1.407 | 0.16 | |
| Mouth breathing | T0 | 2.00 (1.00, 3.00) | 2.00 (1.00, 3.00) | −1.515 | 0.13 |
| T1 | 1.00 (0.00, 2.00) | 1.00 (0.00, 1.50) | −0.900 | 0.37 | |
| T2 | 1.00 (0.00, 2.00) | 0.00 (0.00, 1.00) | −2.650 | 0.008 | |
| Restless sleep | T0 | 2.00 (1.00, 3.00) | 2.00 (1.00, 2.25) | −0.141 | 0.89 |
| T1 | 1.00 (0.00, 1.00) | 1.00 (0.00, 2.00) | −0.157 | 0.88 | |
| T2 | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.00) | −2.759 | 0.006 |
Data are presented as median (interquartile range P25, P75). †, Mann-Whitney U test. SAOS, spleen aminopeptide oral solution; T0, before treatment; T1, after 1 month of treatment; T2, after 2 months of treatment.
Comparison of medication scores between the control group and SAOS group
Based on the treatments used in this study, the additional medication was scored as follows: (I) oral and/or topical antihistamines (nasal or ocular): 1 point per day; (II) nasal corticosteroids: 2 points per day; (III) oral corticosteroids: 3 points per day; (IV) β2 agonists (if asthma was present): 1 point per day; (V) inhaled corticosteroids: 2 points per day. The total medication score is detailed in Supplementary file (Appendix 1). As shown in Table 5, the difference in medication scores between T1 and T0 was not significantly different between the control group and the SAOS group (Z=−1.964, P=0.050). However, the difference in medication scores between T2 and T0 was significantly lower in the SAOS group compared to the control group (Z=−2.299, P=0.02).
Table 5
| Score difference | Control group (n=36) | SAOS group (n=42) | Z | P |
|---|---|---|---|---|
| T1–T0 | 0.00 (0.00, 2.25) | 0.00 (0.00, 0.00) | −1.964 | 0.050† |
| T2–T0 | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | −2.299 | 0.02† |
Data are presented as median (interquartile range P25, P75). †, Mann-Whitney U test. SAOS, spleen aminopeptide oral solution; T0, before treatment; T1, after 1 month of treatment; T2, after 2 months of treatment.
Safety evaluation between the control group and SAOS group
Results from blood routine and urine routine tests were analyzed for safety evaluation. As shown in Table 6, no significant differences were observed in the results of these tests between the control group and the SAOS group at T0 and T2 (P>0.05).
Table 6
| Indices | T0 | T2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control group (n=36) | SAOS group (n=42) | F/Z | P | Control group (n=36) | SAOS group (n=42) | F/Z | P | ||
| Blood routine | |||||||||
| Plt count (109/L) | 290.11±8.45 | 320.14±9.67 | 5.298 | 0.024† | 292.26±55.47 | 318.87±63.93 | 2.875 | 0.09† | |
| WBC count (109/L) | 8.45 (6.75, 9.86) | 8.92 (8.05, 10.70) | −1.363 | 0.173‡ | 7.63±1.67 | 8.35±1.96 | 2.262 | 0.14† | |
| LY count (109/L) | 4.11 (2.95, 5.28) | 3.80 (3.32, 4.76) | −0.301 | 0.764‡ | 3.84 (3.13, 4.65) | 3.65 (3.03, 4.52) | −0.516 | 0.61‡ | |
| GR (%) | 42.76±13.13 | 43.55±10.49 | 0.085 | 0.771† | 41.81±10.72 | 42.91±8.83 | 0.075 | 0.79† | |
| RBC count (1012/L) | 4.55±0.34 | 4.72±0.35 | 4.909 | 0.030† | 4.72±0.65 | 4.73±0.33 | 0.527 | 0.47† | |
| Hb (g/L) | 126.81±8.14 | 129.83±7.69 | 2.846 | 0.096† | 127.58±10.04 | 129.49±7.98 | 0.857 | 0.36† | |
| Hematocrit | 36.30 (34.40, 38.50) | 37.60 (35.65, 39.80) | −2.256 | 0.024‡ | 36.80 (34.50, 39.10) | 37.30 (35.50, 38.95) | −0.728 | 0.47‡ | |
| EOS count | 250.00 (140.00, 480.00) |
380.00 (190.00, 720.00) |
1.537 | 0.124‡ | 250.00 (140.00, 510.00) |
395.00 (207.50, 752.50) |
−1.537 | 0.12‡ | |
| Urine routine [the number of abnormal patient (%)] | |||||||||
| WBC count | 2 (5.6) | 1 (2.4) | – | 0.593§ | 0 (0.0) | 2 (4.8) | – | 0.497§ | |
| RBC count | 3 (8.3) | 1 (2.4) | – | 0.330§ | 1 (2.8) | 2 (4.8) | – | >0.99§ | |
| Protein | 4 (11.1) | 2 (4.8) | – | 0.406§ | 2 (5.6) | 5 (11.9) | – | 0.44§ | |
| Glucose | 0 (0.0) | 0 (0.0) | – | – | 0 (0.0) | 0 (0.0) | – | – | |
| OB | 2 (5.6) | 5 (11.9) | – | 0.442§ | 0 (0.0) | 1 (2.4) | – | >0.99§ | |
| Ketones | 0 (0.0) | 3 (7.1) | – | 0.245§ | 0 (0.0) | 2 (4.8) | – | 0.497§ | |
| Bilirubin | 0 (0.0) | 0 (0.0) | – | 1.000§ | 0 (0.0) | 2 (4.8) | – | 0.497§ | |
| URO | 1 (2.8) | 2 (4.8) | – | 0.650§ | 0 (0.0) | 4 (9.5) | – | 0.12§ | |
Data are presented as n (%), median (interquartile range P25, P75), or mean ± SD. †, ANOVA; ‡, Mann-Whitney U test; §, Fisher’s exact test. SAOS, spleen aminopeptide oral solution; Plt, platelet; WBC, white blood cell; LY, lymphocyte; GR, granulocyte; RBC, ed blood cell; Hb, hemoglobin; EOS, eosinophil; OB, occult blood test; URO, urobilinogen; T0, before treatment; T2, after 2 months of treatment; SD, standard deviation.
Discussion
A total of 100 children were enrolled in this study, with 22 children dropping out due to protocol violations. Based on the PPS dataset, 78 children were included, with 36 in the control group and 42 in the SAOS group. There were no significant differences in baseline A/N ratio, nasal symptom scores, or AH scores between the control and SAOS groups. However, after 8 weeks of treatment, no significant difference in nasal symptom scores between the two groups. The A/N ratio in the SAOS group was significantly lower than in the control group, additionally, some AH scores (mouth breathing and restless sleep) showed significant improvement in the SAOS group. Moreover, SAOS reduced the need for additional medications in children, and no adverse events were observed during 8 weeks of treatment.
Obstructive sleep apnea syndrome is a common issue in the pediatric population, with an incidence of about 2%. Its risk factors include obesity, AH, tonsil hypertrophy, AR, and craniofacial abnormalities (18). Among these, AH is the most significant obstructive factor. Nasal steroids such as Mometasone (15) are prescribed for the treatment of pediatric AH. These medications have been shown to decrease the size of the adenoids and alleviate symptoms of nasal obstruction (6). Oral cysteine leukotriene receptor antagonists, such as Montelukast, are also used to manage AR during inflammatory episodes related to AH (11). Although Montelukast effectively reduce the A/N ratio and obstruction symptoms, it has been associated with an increasing number of neuropsychiatric adverse drug reactions, particularly in children, including depression, sleep disturbance, and suicidal ideation (19). Additionally, several adjunctive therapies are used to treat AH, including nasal irrigation, traditional Chinese medicine, bacteriotherapy, and halotherapy. (I) Nasal irrigation: this involves the mechanical removal of mucus by rinsing the nasal cavity with solutions containing various substances. It is used to alleviate nasal symptoms associated with sinusitis, AR, and viral upper respiratory tract infections, which are closely related to AH (13). The nasal solutions may include isotonic saline, hypertonic saline, and antibacterial agents such as hypochlorous acid and tobramycin. Among these, isotonic saline and hypertonic saline are the most commonly used. Chadha et al. compared hypertonic saline and isotonic saline for treating AH and found that hypertonic saline resulted in a significant decrease (43.5%) in AH, while isotonic saline had no effect on adenoid size (20). (II) Traditional Chinese medicine: recent studies have investigated the effects of herbal extracts used in Chinese medicine on AH. A meta-analysis published in 2019 reviewed the efficacy of Chinese medicine in treating AH in children, including 10 studies with 803 children. The results indicated that the remission rate with Chinese medicine was better than with Western medicine (21). However, these studies involved a wide variety of Chinese medicine compounds, and further research is needed to standardize the application of herbal therapy and ensure its safety. (III) Bacteriotherapy: streptococcus salivarius K12 is an oral probiotic that colonizes the nasopharynx and adenoid tissue in children, inhibiting the growth of pathogens such as Streptococcus pyogenes, Streptococcus pneumoniae, and Moraxella catarrhalis, which cause bacterial pharyngotonsillitis (22,23). The oral form of Streptococcus salivarius K12 has been reported to decrease adenoid size and relieve symptoms associated with otitis media in children (23). (IV) Halotherapy: halotherapy is based on the therapeutic effects of dry-salt inhalation on the upper respiratory tract. Gelardi et al. evaluated the effectiveness of a micronized, iodized-salt aerosol in children aged 4−12 years with AH (24). The results showed a 25% decrease in adenoid and/or tonsillar hypertrophy.
This study provides a novel idea of adjuvant therapy. SAOS, an immunomodulator, is primarily composed of peptides and nucleotides extracted from fresh bovine spleen. SAOS is used to treat cellular immune dysfunction and autoimmune disorders, such as cough variant asthma (16), liver injury (17), and pediatric pneumonia (25). In this study, treatment regimens for both control and SAOS groups were found to improve symptoms of AR. Regarding the symptoms of AR, improvement increased over time, but no statistically significant difference was observed between the two groups. For AH, there was no statistically significant difference in the A/N ratio between the SAOS group and the control group before treatment. However, after 8 weeks of treatment, the A/N ratio in the SAOS group was significantly lower than that in the control group (Z=−3.536, P<0.001). An A/N ratio ranging from 0.61 to 0.70 indicates mild AH, an A/N ratio ranging from 0.71 to 0.80 indicates moderate AH, and an A/N ratio greater than 0.80 indicates severe AH. After 8 weeks of treatment, the proportion of severe and moderate AH in the SAOS group (11.90%) was significantly lower than that in the control group (52.78%). The improvement in adenoid size (A/N ratio) was clinically relevant. For the clinical symptoms of AH (nasal congestion, snoring, mouth breathing, and restless sleep), the SAOS group showed more significant improvements compared to the control group after 8 weeks of treatment. Specifically, the SAOS group had significant improvements in mouth breathing and sleep disturbance (P=0.008 and P=0.006), while there were no significant differences in nasal congestion and snoring between the two groups (P=0.16 and P=0.16). In conclusion, after 8 weeks of treatment, the sizes of the adenoids in the SAOS group decreased significantly, whereas there was no reduction in the control group. The clinical symptoms associated with AH improved more significantly in the SAOS group compared to the control group.
However, after 8 weeks of treatment, the symptoms of AH in the SAOS group were not completely alleviated. Possible reasons may be as following: (I) The children included in this study had experienced multiple infections, such as coronavirus disease 2019 (COVID-19), influenza, and mycoplasma, which may have led to a recurrence of AH. (II) Previous studies have suggested that hypertonic saline solution is more effective for AH in children, while isotonic saline solution has no effect on adenoid size. Our study, consistent with previous research, showed no improvement in the size of adenoids in the control group. More satisfactory results might have been achieved if hypertonic saline solution had been used. (III) Due to the side effects of Montelukast, it was not used in this study and was replaced with Loratadine Syrup, another anti-allergic medication. The results showed that Loratadine Syrup only improved symptoms of AR but did not reduce the size of adenoids. Previous studies have reported that nasal steroids are effective in reducing adenoid size while treating AR (3-6). Therefore, future studies should consider replacing Loratadine Syrup with nasal steroids. (IV) The duration of treatment for AH varies in previous studies, ranging from 8 to 24 weeks (6,13). These studies suggest that treatment for AH is a lengthy process, and 8 weeks of treatment may be insufficient. In summary, improving medications and extending the treatment period in future studies may lead to more significant therapeutic effects.
Our study also investigated whether SAOS could reduce the need for concurrent medication. As anticipated, SAOS, as an immunomodulator, was found to decrease the use of other medications for AR. Regarding the safety of SAOS, it was found to be very safe, with no observed adverse reactions during the 8-week treatment period.
However, there were some limitations in our study. Firstly, although AH is a major cause of obstructive sleep apnea syndrome, we did not conduct polysomnography (PSG) examinations on the children. The primary outcome used in our study was the A/N ratio, primarily due to the complexity and impracticality of PSG examinations in outpatient settings. Secondly, our study was conducted at a single center. Thirdly, as discussed above, improving medications and extending the treatment period in future studies may yield more significant therapeutic effects. Thirdly, A total of 22 children were excluded in this study, including 2 children who were excluded because their A/N ratio was 0.6 before medication, 3 children who voluntarily withdrew from the study, and 13 children who were lost to follow-up during medication monitoring. The primary reason for high drop rate was likely due to the impact of COVID-19, with some children being quarantined at home or in other locations, making it impossible for them to attend follow-up visits on time. Moreover, the taste of SAOS is unpleasant, and children do not like to drunk it. However, despite these protocol violations, the final sample size in the PPS was still sufficient to assess the primary outcome, achieving 95% power. In future, we plan to enhance the treatment regimen by including nasal steroids, hypertonic saline solution, and SAOS, and to extend the treatment period to 3 months to achieve better therapeutic outcomes.
Conclusions
This study established a randomized controlled clinical trial to evaluate the efficacy and safety of SAOS in children with AR and AH. Our study found that SAOS, as an adjuvant therapy for 8 weeks, significantly reduced adenoid size and improved clinical symptoms associated with AH. Additionally, no adverse events related to SAOS were observed during the 8-week treatment period, indicating good safety. However, despite of improvements after 8 weeks of treatment, the clinical symptoms of AH were not completely alleviated. Therefore, future studies should improve the treatment protocol and extend the treatment period to achieve better therapeutic outcomes.
Acknowledgments
We would like to express our sincere gratitude to all those who contributed to this study.
Funding: This research was supported by
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-203/rc
Trial Protocol: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-203/tp
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-203/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-203/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-203/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Children’s Hospital Affiliated with Fudan University (No. 2021-423-04), and informed consent was obtained from the patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nur Husna SM, Tan HT, Md Shukri N, et al. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front Med (Lausanne) 2022;9:874114. [Crossref] [PubMed]
- Ohuche IO, Iloanusi NI, Dike CM, et al. Clinical presentation, radiographic findings, and treatment outcomes in children with adenoid hypertrophy in a paediatric outpatient clinic in Enugu, Nigeria. Ghana Med J 2023;57:204-9. [Crossref] [PubMed]
- Onal M, Elsurer C, Duran T, et al. Possible role of endoplasmic reticulum stress in the pathogenesis of chronic adenoiditis and adenoid hypertrophy: A prospective, parallel-group study. Laryngoscope Investig Otolaryngol 2024;9:e1240. [Crossref] [PubMed]
- Yang Y, Li X, Ma Q, et al. Detecting epidemiological relevance of adenoid hypertrophy, rhinosinusitis, and allergic rhinitis through an Internet search. Eur Arch Otorhinolaryngol 2022;279:1349-55. [Crossref] [PubMed]
- Niedzielski A, Chmielik LP, Mielnik-Niedzielska G, et al. Adenoid hypertrophy in children: a narrative review of pathogenesis and clinical relevance. BMJ Paediatr Open 2023;7:e001710. [Crossref] [PubMed]
- Ahmad Z, Krüger K, Lautermann J, et al. Adenoid hypertrophy-diagnosis and treatment: the new S2k guideline. Adenoide Vegetationen – Diagnostik und Therapie – die neue S2k-Leitlinie. HNO 2023;71:67-72. [Crossref] [PubMed]
- Warman M, Granot E, Halperin D. Improvement in allergic and nonallergic rhinitis: A secondary benefit of adenoidectomy in children. Ear Nose Throat J 2015;94:220;222;224-7.
- Zhang H, Sun Y, Shen C, et al. Evaluation Value of Allergy in Adenoid Hypertrophy Through Blood Inflammatory Cells and Total Immunoglobulin E. Pediatr Allergy Immunol Pulmonol 2022;35:139-44. [Crossref] [PubMed]
- Cho KS, Kim SH, Hong SL, et al. Local Atopy in Childhood Adenotonsillar Hypertrophy. Am J Rhinol Allergy 2018;32:160-6. [Crossref] [PubMed]
- Alanazi F, Alruwaili M, Alanazy S, et al. Efficacy of montelukast for adenoid hypertrophy in paediatrics: A systematic review and meta-analysis. Clin Otolaryngol 2024;49:417-28. [Crossref] [PubMed]
- Zwierz A, Domagalski K, Masna K, et al. Maximal medical treatment of adenoid hypertrophy: a prospective study of preschool children. Eur Arch Otorhinolaryngol 2024;281:2477-87. [Crossref] [PubMed]
- Jafari M, Pourroshani B, Eftekhari K, et al. Effect of Combination Montelukast and Nasal Mometasone on Childhood Adenoid Hypertrophy. Iran J Otorhinolaryngol 2024;36:391-7. [Crossref] [PubMed]
- Çetkin M, Çetkin HE. Adjunctive Treatment of Pediatric Adenoidal Hypertrophy: A Review. Altern Ther Health Med 2023;29:46-53.
- Sui H, Zhang H, Ding W, et al. Effective treatment of a child with adenoidal hypertrophy and severe asthma by omalizumab: a case report. Allergy Asthma Clin Immunol 2022;18:94. [Crossref] [PubMed]
- Eldegeir M, Marry NA, Awami F, et al. The combination of nasal steroids and anti-leukotriene to reduce adenectomy in children with OSA and adenoid hypertrophy. Qatar Med J 2023;2023:31. [Crossref] [PubMed]
- Liu JT, Yang J, Guo R, et al. Effect of Spleen Aminopeptide Oral Lyophilized Powder and Fluticasone/salmeterol Powder Inhaler on Pulmonary Function and Incidence of Adverse Reactions in Children with Cough Variant Asthma. Altern Ther Health Med 2024;AT9521.
- Wu Y, Dong X, Wu R, et al. Efficacy and safety of spleen aminopeptide oral lyophilized powder in ameliorating liver injury in infants and children with human cytomegalovirus infection: a single-center study in China. Transl Pediatr 2021;10:136-45. [Crossref] [PubMed]
- Qian Y, Dharmage SC, Hamilton GS, et al. Longitudinal risk factors for obstructive sleep apnea: A systematic review. Sleep Med Rev 2023;71:101838. [Crossref] [PubMed]
- Iacobucci G. Montelukast: UK regulator says asthma drug needs clearer warnings of side effects. BMJ 2024;385:q1048. [Crossref] [PubMed]
- Chadha NK, Zhang L, Mendoza-Sassi RA, et al. Using nasal steroids to treat nasal obstruction caused by adenoid hypertrophy: does it work? Otolaryngol Head Neck Surg 2009;140:139-47. [Crossref] [PubMed]
- Wang P, Kong W, Shan Y. The efficacy and safety of Chinese herbal compound or combined with western medicine for pediatric adenoidal hypertrophy: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22023. [Crossref] [PubMed]
- Power DA, Burton JP, Chilcott CN, et al. Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12. Eur J Clin Microbiol Infect Dis 2008;27:1261-3. [Crossref] [PubMed]
- Di Pierro F, Di Pasquale D, Di Cicco M. Oral use of Streptococcus salivarius K12 in children with secretory otitis media: preliminary results of a pilot, uncontrolled study. Int J Gen Med 2015;8:303-8. [Crossref] [PubMed]
- Gelardi M, Iannuzzi L, Greco Miani A, et al. Double-blind placebo-controlled randomized clinical trial on the efficacy of Aerosal in the treatment of sub-obstructive adenotonsillar hypertrophy and related diseases. Int J Pediatr Otorhinolaryngol 2013;77:1818-24. [Crossref] [PubMed]
- Yue Y, Liang Q, Shi L, et al. Effect of comprehensive nursing intervention on the efficacy of spleen aminopeptide combined with aerosol inhalation in the treatment of pediatric pneumonia. Pak J Med Sci 2023;39:1086-90. [Crossref] [PubMed]


