
Prognostic factors and surgical management in pediatric primary lung cancer: a retrospective cohort study using SEER data
Highlight box
Key findings
• Surgical intervention, particularly lobectomy, plays a crucial role in management, with histological classification of pediatric lung cancer (LC), Surveillance, Epidemiology, and End Results stage, surgery, and tumor size identified as independent prognostic factors guiding personalized treatment decisions for pediatric LC.
What is known and what is new?
• Surgical intervention significantly improves survival rates for patients with American Joint Committee on Cancer stages II and III, those with stage IV do not derive significant survival benefits. The extent of lymph node dissection should be tailored according to disease stage, with no significant survival advantage observed in early-stage disease.
• Tumor size significantly influences the choice of surgical approach, with lobectomy and local tumor excision showing better outcomes for tumors smaller than 5 cm in diameter. However, larger tumors may not exhibit significant survival differences based on the surgical modality.
What is the implication, and what should change now?
• Our findings underscore the importance of personalized treatment strategies in pediatric LC, informed by histology, disease stage, and tumor size, highlighting the necessity for further prospective studies to validate these conclusions and explore emerging therapeutic modalities.
Introduction
Lung cancer (LC) has increased mortality and morbidity rates worldwide (1,2). According to the data published by the American Cancer Society in 2023, LC ranked 2nd among all new cancer (accounting for ~12%) cases and 1st major cause of death by cancer (21%). Early-stage LC is mostly asymptomatic, therefore most patients are already in the advanced stage at the time of medical treatment after symptom onset, and the 5-year overall survival (OS) rate of advanced LC patients is ~20% (3-5). However, in children, primary LCs are rare with an incidence rate of <1/1,000,000 per year, and account for <0.2% of all pediatric cancers, making diagnosis and evidence-based treatment decisions very difficult (6-8).
A review of past literature on pediatric LC reveals that most studies are based on case reports and case series (9,10). Research specifically focusing on the prognosis of pediatric LC is limited, with very few studies available. Histology, surgery, tumor site, and stage have been identified as key prognostic factors influencing the outcomes of pediatric LC (11,12). Currently, the treatment strategy for pediatric LC patients involves referring to the standards for adults and developing treatment plans after discussing with adult LC patients oncologists (13). Surgical treatment plays a very important role in the comprehensive treatment of LC in both adults and children (14). However, because of the low incidence rate, analyzing the effect and outcome of surgical treatment of pediatric LC patients in a single medical center is difficult. Furthermore, studies discussing surgical strategies and their benefits for pediatric LC patients with different clinical characteristics remain limited. Therefore, to address the aforementioned limitations, this research employed the Surveillance, Epidemiology, and End Results (SEER) database, which offers an extensive dataset on various cancers in the American population.
This retrospective study collected general clinical information, surgical treatment, and prognostic data of 337 pediatric primary LC patients (aged =0–19 years) from the SEER database between 1988 and 2019 to analyze the associated prognostic factors. Furthermore, the survival benefit of surgical treatment among patients with different clinical characteristics was also assessed. Per our knowledge, this is the largest population-based study of primary malignant lung tumors in children to date. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-174/rc).
Methods
Patient selection
The data utilized in this research were acquired from the SEER database, which comprises 18 population-based cancer registries and covers above 28% of the American population (15). This study leveraged SEER-Stat version 8.4.2, which offers extensive data on morphological and histological codes, patient demographics, stage, treatment methods, and patient survival (16,17). The robustness of the SEER database ensures that our findings are based on a large and diverse patient population.
Information on diagnosed LC patients was extracted based on the following inclusion criteria: (I) site recode International Classification of Disease for Oncology, 3rd edition (ICD-O-3)/World Health Organization (WHO) 2008 = Lung and Bronchus; (II) diagnosed between 1988 and 2019; (III) age at diagnosis between 0 to 19 years; (IV) complete follow-up. Individuals with multiple primary cancers or unknown survival time were excluded from the research. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Variable collection
Data on different demographic variables including race, year of diagnosis, age, primary site, laterality, gender, tumor size, use of surgery, surgical methods, and histology were acquired. Furthermore, American Joint Committee on Cancer (AJCC) stage and survival time data were also harvested. Tumor size was categorized into two groups based on the optimal cut-off points determined using the X-tile program (Figure 1), with <5 and ≥5 cm size. The end date of follow-up for the SEER cohort was December 31, 2019. The OS was defined as the duration from diagnosis until the last follow-up or death by any cause.

Statistical analysis
The data are illustrated as frequencies (in %) or median (Q1, Q3). The demographics and disease features of different patient groups were compared using a chi-square test and Fisher’s exact test for categorical variables. The rank sum test was employed to compare quantitative data with skewed distribution between the groups. Furthermore, survival curves were generated with the help of the Kaplan-Meier method, while for comparing the strata, a log-rank test was performed. Univariable analysis was conducted to identify which among those confounders were potential prognostic factors. Variables which were of statistical significance (P<0.05) in the univariable Cox regression model were subsequently incorporated into the multivariable Cox proportional hazards regression analysis. For data processing and analysis, R version 4.3.0 (2023-04-21) was utilized in conjunction with the Storm Statistical Platform (www.medsta.cn/software). A two-sided P value of <0.05 was set as the threshold for statistically significant differences.
Results
General information
This research included data on 337 children ≤19 years old with primary LC, acquired from the SEER database between 1988 and 2019. The children indicated a median age of 15 years and an average OS of 68 months. The female-to-male ratio was close to 1:1. The most common pathological type was carcinoid tumor (31.45%), followed by pulmonary/pleuropulmonary blastoma (21.07%) and mucoepidermoid carcinoma (12.17%). Most of the tumors were <5 cm in diameter (63.79%) or confined in situ (46.77%). Furthermore, approximately 80% of all pediatric LC patients underwent surgical treatment. Moreover, lobectomy (30.33%) was the predominant surgical modality employed, followed by excision or resection of less than one label, or local tumor destruction (ERL) (23.12%), lobectomy with dissection of lymph nodes (LDLN) (17.72%), and pneumonectomy (8.41%). In addition, regional lymph node surgery was performed in 51.16% of cases (Table 1).
Table 1
| Variable | Value (n=337) |
|---|---|
| Overall survival (months) | 68.00 (13.00, 152.00) |
| Age (years) | 15.00 (6.00, 18.00) |
| Sex | |
| Female | 171 (50.74) |
| Male | 166 (49.26) |
| Year of diagnosis | |
| 1988–1999 | 43 (12.76) |
| 2000–2009 | 142 (42.14) |
| 2010–2019 | 152 (45.1) |
| Race | |
| Black | 45 (13.35) |
| White | 268 (79.53) |
| Other/unknown | 24 (7.12) |
| Primary site | |
| Lower lobe | 112 (33.23) |
| Main bronchus | 39 (11.57) |
| Middle lobe | 31 (9.2) |
| Overlapping lesion | 16 (4.75) |
| Upper lobe | 97 (28.78) |
| Unknown | 42 (12.46) |
| ICD-O-3 | |
| Carcinoid tumor | 106 (31.45) |
| Pulmonary/pleuropulmonary blastoma | 71 (21.07) |
| Mucoepidermoid carcinoma | 41 (12.17) |
| Adenocarcinoma | 19 (5.64) |
| Other | 100 (29.67) |
| Laterality | |
| Left | 144 (42.73) |
| Right | 177 (52.52) |
| Bilateral/unknown | 16 (4.75) |
| Type of resection | |
| ERL | 77 (23.12) |
| LDLN | 59 (17.72) |
| Lobectomy | 101 (30.33) |
| Pneumonectomy | 28 (8.41) |
| No | 68 (20.42) |
| Tumor size | |
| <5 cm | 155 (63.79) |
| ≥5 cm | 88 (36.21) |
| N stage | |
| N0 | 144 (61.80) |
| N1 | 20 (8.58) |
| N2 | 29 (12.45) |
| N3 | 12 (5.15) |
| NX | 28 (12.02) |
| AJCC stage | |
| I | 86 (41.95) |
| II | 41 (20.00) |
| III | 23 (11.22) |
| IV | 55 (26.83) |
| Surgery | |
| No | 68 (20.18) |
| Yes | 269 (79.82) |
| Regional lymph node surgery | |
| No | 148 (48.84) |
| Yes | 155 (51.16) |
| SEER stage | |
| Distant | 78 (24.00) |
| Localized | 152 (46.77) |
| Regional | 95 (29.23) |
Data are presented as median (Q1, Q3) or n (%). ICD-O-3, international classification of disease for oncology, 3rd edition; ERL, excision or resection of less than one label, or local tumor destruction; LDLN, lobectomy with dissection of lymph nodes; AJCC, American Joint Committee on Cancer; SEER, the surveillance, epidemiology, and end results database.
Analysis of prognostic-related factors
For all children and adolescents, the 1-, 3-, 5-, and 10-year OS was 88.5%, 78.0%, 77.6%, and 73.7%, respectively. The uni- and multivariate Cox regression revealed four independent prognostic factors for pediatric primary LC, including pathologic classification, SEER stage, surgery, and tumor size (Table 2). Figure 2 indicates the survival curve of different surgery, SEER stages, histological types, and tumor size. The OS of mucoepidermoid carcinoma, carcinoid tumors, and pulmonary/pleuropulmonary blastoma were in the top three, and patients with these indicated significantly better OS than those with other histology.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Age | 1.01 (0.98–1.05) | 0.57 | |||
| Sex | |||||
| Female | Reference | ||||
| Male | 1.00 (0.64–1.56) | >0.99 | |||
| Year of diagnosis | |||||
| 1988–1999 | Reference | Reference | |||
| 2000–2009 | 0.62 (0.36–1.10) | 0.10 | 1.20 (0.60–2.39) | 0.60 | |
| 2010–2019 | 0.46 (0.24–0.88) | 0.02 | 0.92 (0.40–2.15) | 0.85 | |
| Race | |||||
| White | Reference | ||||
| Black | 1.18 (0.62–2.24) | 0.62 | |||
| Other/unknown | 1.56 (0.71–3.41) | 0.27 | |||
| Primary site | |||||
| Upper lobe | Reference | Reference | |||
| Lower lobe | 1.12 (0.59–2.13) | 0.73 | 1.14 (0.55–2.36) | 0.73 | |
| Middle lobe | 0.90 (0.33–2.47) | 0.84 | 1.51 (0.44–5.13) | 0.51 | |
| Main bronchus | 0.65 (0.24–1.78) | 0.41 | 0.77 (0.26–2.34) | 0.65 | |
| Overlapping lesion | 2.33 (0.91–5.96) | 0.08 | 2.18 (0.74–6.42) | 0.16 | |
| Unknown | 4.13 (2.19–7.80) | <0.001 | 0.95 (0.41–2.19) | 0.91 | |
| ICD-O-3 | |||||
| Carcinoid tumor | Reference | Reference | |||
| Pulmonary/pleuropulmonary blastoma | 9.86 (2.85–34.13) | <0.001 | 6.41 (1.69–24.35) | 0.006 | |
| Adenocarcinoma | 29.55 (8.10–107.89) | <0.001 | 8.82 (2.20–35.25) | 0.002 | |
| Mucoepidermoid carcinoma | 0.84 (0.09–8.08) | 0.88 | 1.83 (0.17–20.03) | 0.62 | |
| Other | 25.19 (7.84–80.93) | <0.001 | 8.03 (2.37–27.20) | <0.001 | |
| Laterality | |||||
| Left | Reference | Reference | |||
| Right | 1.19 (0.73–1.92) | 0.49 | 1.42 (0.77–2.60) | 0.26 | |
| Bilateral/unknown | 4.04 (1.96–8.33) | <0.001 | 1.15 (0.41–3.18) | 0.79 | |
| SEER stage | |||||
| Regional | Reference | Reference | |||
| Localized | 0.09 (0.03–0.29) | <0.001 | 0.15 (0.04–0.56) | 0.005 | |
| Distant | 4.98 (2.97–8.35) | <0.001 | 1.76 (0.77–4.00) | 0.18 | |
| Unknown | 0.36 (0.05–2.69) | 0.32 | 0.27 (0.03–2.27) | 0.23 | |
| Surgery | |||||
| Yes | Reference | Reference | |||
| No | 7.45 (4.75–11.67) | <0.001 | 2.05 (1.13–3.72) | 0.02 | |
| Regional lymph node surgery | |||||
| No | Reference | Reference | |||
| Yes | 0.55 (0.33–0.92) | 0.02 | 0.93 (0.49–1.73) | 0.81 | |
| Unknown | 1.45 (0.81–2.61) | 0.21 | 1.18 (0.61–2.27) | 0.62 | |
| AJCC stage | |||||
| I | Reference | Reference | |||
| II | 27.83 (3.59–215.62) | 0.001 | 3.18 (0.34–29.40) | 0.31 | |
| III | 39.61 (5.02–312.69) | <0.001 | 2.09 (0.21–21.09) | 0.53 | |
| IV | 109.01 (14.92–796.36) | <0.001 | 2.65 (0.27–26.25) | 0.41 | |
| Unknown | 13.29 (1.78–99.09) | 0.01 | 1.96 (0.21–18.39) | 0.56 | |
| Tumor size | |||||
| <5 cm | Reference | Reference | |||
| ≥5 cm | 8.68 (4.14–18.19) | <0.001 | 2.87 (1.20–6.89) | 0.02 | |
| Unknown | 7.90 (3.80–16.41) | <0.001 | 2.46 (1.01–6.05) | 0.049 | |
HR, hazards ratio; CI, confidence interval; ICD-O-3, International Classification of Disease for Oncology, 3rd edition; SEER, Surveillance, Epidemiology, and End Results; AJCC, American Joint Committee on Cancer.

Patients with AJCC stage IV or distant-stage disease did not indicate significant survival benefit from surgery
Although the AJCC stage was not an independent factor influencing the prognosis of pediatric primary LC in multifactorial Cox regression. However, AJCC staging is an important guide in clinical diagnostic and treatment decisions. Therefore, the survival curves were used to assess the survival benefit of surgical treatment at different AJCC stages. Furthermore, it was revealed that AJCC stage IV patients had the worst prognosis, while stage I indicated the best prognosis. Moreover, the difference in survival between stage II and III was not significant [hazards ratio (HR): 2.93; 95% confidence interval (CI): 2.21–3.87; P<0.001; Figure 3A]. For AJCC stage I, the choice of different surgical modalities did not significantly affect the patient’s survival (P=0.22; Figure 3B). In addition, for AJCC stages II and III children, those who underwent lobectomy with or without lymph node dissection had significantly prolonged survival than those who did not undergo surgical intervention (HR: 1.52; 95% CI: 1.07–2.15; P=0.02; Figure 3C). For AJCC stage IV, there was no significant difference in survival between the children who underwent surgical intervention and those who did not (P=0.58; Figure 3D). Furthermore, the survival of children with distant-stage disease who received surgical intervention was better than those without surgery, but the difference was not statistically significant (HR: 0.59; 95% CI: 0.33–1.03; P=0.06; Figure 3E). The survival time of children with localized-stage disease who underwent ERL, lobectomy, or LDLN mono-treatment was markedly higher than those who did not undergo surgical intervention (Figure 3F).
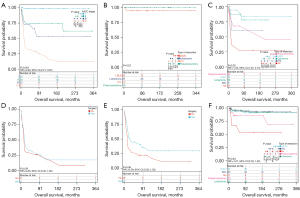
Patients with AJCC stage I and II or localized-stage disease did not indicate significant survival benefit from lymph node dissection
Whether lymph node dissection is required is a controversial question for surgeons. Here, survival curves were employed to explore the difference in survival between clinical subgroups in terms of whether lymph node dissection should be performed. It was found that surgical intervention with lymph node dissection did not notably improve survival in children with AJCC stage I and II (P=0.27) or N stage 0 (P=0.10) or localized-stage disease (P=0.68) (Figure 4A-4C). However, surgical intervention with lymph node dissection significantly improved the survival of children with AJCC stages III and IV (P=0.02) or regional lymph node metastases (P=0.02) or distant-stage disease (P=0.04) (Figure 4D-4F).

Patients with tumor diameter less than 5 cm have significantly increased survival after ERL and lobectomy
Tumor size is also a key factor in determining the choice of surgical approach. The survival analysis indicated that surgery could markedly improve the survival of children with tumor diameters <5 (P=0.008) or ≥5 cm (P<0.001) (Figure 5A). When the tumor diameter was <5 cm, the survival time of patients who underwent ERL (P=0.03) and lobectomy (P<0.001) was significantly higher compared with those who did not undergo surgical intervention, while the survival time of the patients who underwent lobectomy was notably enhanced than the patients who underwent LDLN (P=0.03) and pneumonectomy (P=0.01) (Figure 5B). For children without distant metastasis and with tumor diameter <5 cm, no significant difference was observed in survival after different surgical methods (Figure 5C). However, for patients without distant metastasis and with tumor diameter ≥5 cm, the survival rate after ERL(P=0.13) and lobectomy (P=0.12) was better than that of patients treated with pneumonectomy, but the difference was not statistically significant (Figure 5D).

Discussion
Primary LC is very rare in children with an incidence rate of only 0.03% among all LC cases. Furthermore, the number of pediatric LC patient cases is much lower than that of lung metastases of other childhood malignancies (18). The ratio of primary benign to primary malignant to secondary malignant neoplasms is 1.4:1:11.6 (19). Therefore, because of the unique challenges of this malignancy, comprehensive data and evidence-based treatment guidelines for pediatric LC patients are still lacking. This investigation addressed this research gap by using the SEER dataset to analyze the clinical data of 337 children with primary LC and focusing on the efficiency of surgical treatment in the prognosis of these patients. The data identified four independent prognostic factors in pediatric LC patients, including histological classification, SEER stage, surgery, and tumor size.
In adults, the common LC histological types primarily include squamous cell carcinoma, adenocarcinoma, large cell carcinoma, and small cell carcinoma; however, these are relatively rare in children (11,20). Here, it was revealed that carcinoid tumors were the most common histological subtype of pediatric primary LC patients, followed by pulmonary/pleuropulmonary blastoma and mucoepidermoid carcinoma, consistent with the previous reports (21). Furthermore, it was revealed that the prognosis of the three most common pathological types of pediatric LC patients was good and significantly better than the other histological types. However, a quarter of patients indicated distant metastases at diagnosis, and the 3-year OS was only 35.6%. Moreover, no substantial difference was observed in survival between primary LC patients with distant metastases who did or did not receive surgical intervention. Much research has been conducted for decades to achieve early diagnosis and LC treatment by screening to reduce related mortality (15). Current risk factors for LC in adults include advanced age, history of smoking, history of occupational carcinogen exposure, genetic mutations, and history of chronic lung disease (12,22). Experts around the world suggest that high-risk people should be screened for LC. However, early screening for pediatric LC is difficult due to its great rarity, unspecific symptoms, and lack of relevant risk factor analysis data. It is currently believed that childhood lung malignancies may be associated with underlying congenital lesions, such as cystic adenomatoid malformations (23). Therefore, both the removal of lesions and the examination of the tissue should be considered to confirm the elimination of all abnormal areas. Additionally, persistent symptoms of respiratory infections and coughing up blood should prompt a comprehensive diagnostic process involving imaging and possibly bronchoscopy (20).
Surgical treatment, a cornerstone of LC management in adults, plays a significant role in pediatric cases as well (24). Here, about 80% of pediatric primary LC patients underwent surgery, with lobectomy being the most common surgical procedure. For choosing the extent of surgical resection, although lobectomy is still considered the gold standard for surgical treatment of stage I LC, this research indicated that stage I pediatric LC patients who underwent segmental resection or wedge resection had comparable long-term survival rates to those who underwent lobectomy. Therefore, in pediatric primary LC patients, the extent of resection should be appropriately reduced when necessary for safety reasons. Furthermore, total pneumonectomy should be avoided as much as possible, as it has been associated with an elevated rate of postoperative complications and risk of death in elderly LC patients (25,26). Moreover, this investigation also suggested that in AJCC stages II and III pediatric LC patients, surgical intervention, particularly lobectomy, significantly improves the survival rate. However, for children with AJCC stage IV, surgery did not provide a significant survival benefit. Currently, for treating stage IV pediatric LC patients, a treatment regimen for adult LC patients is referred, the principle of comprehensive treatment based on systemic therapy is adopted, and an individualized treatment strategy is developed according to the patient’s pathological type, molecular genetics characteristics, and organismal status.
Whether lymph node dissection should be performed for treating LC remains a controversial issue. Here, 51.2% (155/303) of the patients had a lymphadenectomy. Furthermore, the long-term survival rate of children with AJCC stage III–IV or regional lymph node metastases substantially increased after lymph node dissection compared to those without lymph node dissection. However, in children with AJCC stage I–II or those without lymph node involvement, lymph node dissection had no significant effect on survival. In addition, it was observed that the extent of lymph node dissection can be tailored according to the patient’s clinical stage. In studies of adult LC patients, lymph node dissection did not indicate a significant postoperative survival advantage over stage I–III LC patients without lymph node metastasis detected by preoperative standardized mediastinal lymph node staging. However, lymph node dissection is still recommended for those who do not have preoperative clear mediastinal lymph node staging (27).
Tumor size is a key factor in determining an appropriate surgical strategy. Here, it was indicated that surgery substantially improved the survival of pediatric LC patients regardless of whether the tumor diameter was greater or smaller than 5 cm. When the tumor diameter was <5 cm, children who underwent ERL, lobectomy, and LDLN indicated markedly prolonged survival time than those who did not undergo surgical intervention. In addition, children who received lobectomy had a notably longer survival time than those who underwent LDLN and pneumonectomy. Although no significant difference was observed in survival rates between surgical modalities in children without distant metastases and with tumors ≥5 cm in diameter, patients who received ERL and lobectomy had better survival than those who underwent pneumonectomy. These data highlight the importance of surgical interventions based on specific clinical features of the disease, including disease stage, tumor size, and lymph node involvement.
Although this study has the largest sample size, there are still certain limitations. First, because of the retrospective nature of the SEER database, particularly the inability to distinguish subtypes within diagnoses, selection bias could not be avoided. Therefore, further prospective studies and clinical trials are required to confirm the findings and provide stronger evidence for treatment recommendations for pediatric primary LC patients. Furthermore, while our study provides broad insights into surgical outcomes, it should not replace individualized clinical decision-making. The role of comprehensive diagnostic evaluations remains paramount in guiding surgical decisions. Second, the available chemotherapy and radiotherapy data do not encompass details such as whether the treatment is neoadjuvant or adjuvant, the specific regimen used, or the dosage administered. Thus, although this research explores the prognostic surgical differences between different clinical subgroups, it lacks an analysis of the synergistic effects of chemotherapy and radiotherapy with surgical treatment. Moreover, the development of targeted- and immune therapies may provide new therapeutic options for these rare tumors, and their role in pediatric LC deserves further investigation.
Conclusions
In summary, pediatric primary LC is a very rare and heterogeneous disease with different histological subtypes. This research highlights the significance of individualized treatment strategies based on specific features of the disease, such as histology, disease stage, lymph node involvement, and tumor size. Furthermore, this investigation provides valuable evidence for clinicians for the management of pediatric primary LC and further research in this challenging area.
Acknowledgments
The authors are grateful to the SEER database for providing the raw research data.
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-174/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-174/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-174/coif). All authors report that this study was supported by scientific research project of Fujian Children’s Hospital (grant number: ETK2023001). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. Lancet 2021;398:535-54. [Crossref] [PubMed]
- Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol 2023;20:624-39. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- Kratzer TB, Bandi P, Freedman ND, et al. Lung cancer statistics, 2023. Cancer 2024;130:1330-48. [Crossref] [PubMed]
- Rossi G, Graziano P. Lung cancer diagnosis. J Xiangya Med 2022;7:16.
- Ferrari A, Brecht IB, Gatta G, et al. Defining and listing very rare cancers of paediatric age: consensus of the Joint Action on Rare Cancers in cooperation with the European Cooperative Study Group for Pediatric Rare Tumors. Eur J Cancer 2019;110:120-6. [Crossref] [PubMed]
- Youlden DR, Foresto SA, Aitken JF. Primary malignant lung tumors in children: A report from the Australian Childhood Cancer Registry, 1983-2015. Pediatr Pulmonol 2020;55:719-22. [Crossref] [PubMed]
- Balzer BWR, Loo C, Lewis CR, et al. Adenocarcinoma of the Lung in Childhood and Adolescence: A Systematic Review. J Thorac Oncol 2018;13:1832-41. [Crossref] [PubMed]
- Zhang M, Song P, Yang L, et al. Primary pulmonary epithelioid malignant peripheral nerve sheath tumor in a child: a case report. Ann Transl Med 2020;8:403. [Crossref] [PubMed]
- Voggel S, Abele M, Seitz C, et al. Primary lung carcinoma in children and adolescents - Clinical characteristics and outcome of 12 cases from the German registry for rare paediatric tumours (STEP). Lung Cancer 2021;160:66-72. [Crossref] [PubMed]
- Shao W, Liu J, Li B, et al. Primary lung cancer in children and adolescents: Analysis of a surveillance, epidemiology, and end results database. Front Oncol 2023;13:1053248. [Crossref] [PubMed]
- Rojas Y, Shi YX, Zhang W, et al. Primary malignant pulmonary tumors in children: a review of the national cancer data base. J Pediatr Surg 2015;50:1004-8. [Crossref] [PubMed]
- Abele M, Bajčiová V, Wright F, et al. Primary lung carcinoma in children and adolescents: An analysis of the European Cooperative Study Group on Paediatric Rare Tumours (EXPeRT). Eur J Cancer 2022;175:19-30. [Crossref] [PubMed]
- Honguero-Martínez AF. Lung cancer surgery at present and tendency. Transl Cancer Res 2022;11:4220-2. [Crossref] [PubMed]
- Cronin KA, Ries LA, Edwards BK. The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute. Cancer 2014;120:3755-7. [Crossref] [PubMed]
- Surveillance Research Program, National Cancer InstituteSEER*Stat software, version 8.4.2. Available online: www.seer.cancer.gov/seerstat
- Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev 1999;8:1117-21.
- Dishop MK, Kuruvilla S. Primary and metastatic lung tumors in the pediatric population: a review and 25-year experience at a large children's hospital. Arch Pathol Lab Med 2008;132:1079-103. [Crossref] [PubMed]
- Davri A, Birbas E, Kanavos T, et al. Deep Learning for Lung Cancer Diagnosis, Prognosis and Prediction Using Histological and Cytological Images: A Systematic Review. Cancers (Basel) 2023;15:3981. [Crossref] [PubMed]
- Adams SJ, Stone E, Baldwin DR, et al. Lung cancer screening. Lancet 2023;401:390-408. [Crossref] [PubMed]
- Shukuya T, Takahashi K, Shintani Y, et al. Epidemiology, risk factors and impact of cachexia on patient outcome: Results from the Japanese Lung Cancer Registry Study. J Cachexia Sarcopenia Muscle 2023;14:1274-85. [Crossref] [PubMed]
- Sidorenkov G, Stadhouders R, Jacobs C, et al. Multi-source data approach for personalized outcome prediction in lung cancer screening: update from the NELSON trial. Eur J Epidemiol 2023;38:445-54. [Crossref] [PubMed]
- Neville HL, Hogan AR, Zhuge Y, et al. Incidence and outcomes of malignant pediatric lung neoplasms. J Surg Res 2009;156:224-30. [Crossref] [PubMed]
- Frederiksen JG, Christensen TD, Petersen RH. Lung cancer surgery in Denmark. J Thorac Dis 2022;14:3638-47. [Crossref] [PubMed]
- Speicher PJ, Ganapathi AM, Englum BR, et al. Survival in the elderly after pneumonectomy for early-stage non-small cell lung cancer: a comparison with nonoperative management. J Am Coll Surg 2014;218:439-49. [Crossref] [PubMed]
- Onaitis MW, Furnary AP, Kosinski AS, et al. Prediction of Long-Term Survival After Lung Cancer Surgery for Elderly Patients in The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2018;105:309-16. [Crossref] [PubMed]
- Dunne EG, Fick CN, Jones DR. Mediastinal Staging in Non-Small-Cell Lung Cancer: Saying Goodbye to Mediastinoscopy. J Clin Oncol 2023;41:3785-90. [Crossref] [PubMed]


