Proximal ureteral atresia, a rare congenital anomaly—incidental finding: a case report
Introduction
Ureteral atresia represents an extremely rare congenital malformation of the ureter, which usually is associated with a dysplastic kidney. The development of ureteral atresia is thought to result from relative or total ischemia that might occur with the migration of the developing kidney with changes in distribution of regional blood supply to the ureter. This theory is based on experimental studies on intestinal atresia, and might be extrapolated to explain ureteral stenosis and postnatal involution of multicystic dysplastic kidney (MDK) (1). It is also hypothesized that the atresia could be caused by a failure of canalization of a segment of ureter during the development and elongation of the ureteric bud: in detail by a failure of Chwalla’s membrane reabsorption. Canalization starts from the midportion and extend cranially and caudally after 40th day. This process normally takes place between the 37th and the 47th days of pregnancy. The atresia may be unilateral or bilateral, short or long and may involve any part of the ureter with the distal one more frequently affected (2,3).
Case presentation
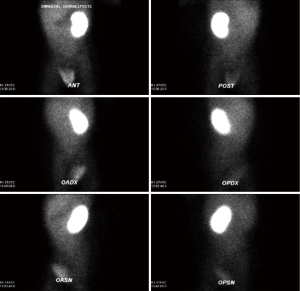
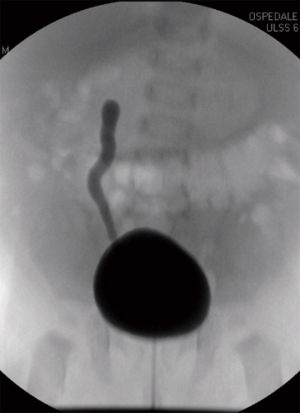
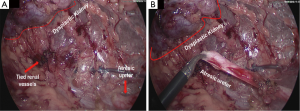
A new-born boy was referred to our department with a prenatal diagnosis of right MDK and left hydroureteronephrosis. He was evaluated by ultrasonographic studies (US) at birth and at 1 month and 2 months old. The technetium-labeled-dimercapto-succinic-acid (DMSA) scintigraphy at 4-month showed the left kidney orthotopic of normal size and morphology without significant pathological defects in uptaking the radionuclide. No uptake in right kidney was recorded (Figure 1). Voiding cysto-urethrogram at 6-month showed a right vesicoureteral reflux (VUR) of II–III grade (Figure 2). In the same period, an US was repeated showing right kidney occupied by multiple cysts, the most voluminous of 13 mm, without any parenchymal cortico-medullary differentiation. The left kidney appeared regular for morphology and size with an ectasia of 6–7 mm of the ureter in paravesical space. Suspecting a primitive obstructive megaureter a dimercapto-acetyl-triglycine scintigraphy (MAG3) scan was performed showing a left pielo-ureteral stasis with good response to the diuretic test without significative signs of obstruction. The imaging studies so concluded for a MDK with associated VUR. The boy presented a regular renal function but was hospitalized twice for suspected pyelonephritis between the 8th and the 10th month of life. Were recorded occasional mild changes in blood pressure. Antibiotic prophylaxis was administered until surgery. When he was 10-month old, he underwent retroperitoneoscopic nephroureterectomy. In operatory room: the patient was placed on left lateral decubitus; Foley’s catheterization of bladder was performed initially before starting the procedure. A subcostal open approach under the 12th rib, over the posterior axillary line, was performed creating a working space in the retroperitoneum with insertion initially of gauzes, then of a 5 mm diameter balloon cannula. A 5-mm 0° or 30° angled lens camera is inserted and under direct vision two 3-mm trocars were placed one anterior and one posterior to the camera to allow an ideal triangulation. Retro-pneumoperitoneum is induced by insufflating CO2 at the minimal pressure to obtain an acceptable operative space (pressure range, 4–5 mmHg). We proceed to the opening of Gerota’s fascia and kidney dissection from the perirenal fat. The vascular pedicle, two arterial branches and one vein, which appeared thin and hypotrophic was identified; the vessels were tied and then dissected. The kidney appeared of small size, irregular in morphology for the presence of multiple cysts; the pelvis was not particularly dilated. Following the lower margin of the psoas muscle looking for the ureter, it was identified few centimetres below the kidney and separate from the pelvis: an atresia of the upper third of ureter (Figure 3). The right ureter was then dissected and isolated just until the paravesical space; then it was tied with stiches and cut. A drain was positioned for 2 days. The patient was discharged in 3th postoperative days.
Discussion
Ureteral atresia, one of the rarest congenital anomalies, represents a failure of the ureter to communicate with the bladder. It is commonly associated with a non-functioning kidney/dysplastic kidney.
The treatment of MCDK has passed through different phases. Historically (until 1980) nephrectomy was long regarded as the standard treatment and the safest way to avoid the complications of infection, bleeding, flank pain, hypertension and possible malignant transformation. In the last decade, there has been a shift in the management of MCDK from surgical removal to a conservative approach using serial US examination. This change in treatment is due to the perception that malignant transformation is a rare occurrence, and to the result of sequential ultrasound imaging of a large number of infants diagnosed with MCDK revealing that most of these structures involute over time. In recent years, the argument has emerged again with regard to the management of MCDK; several studies have recommended surgical removal because the natural history of the condition in the long term is still uncertain, and nephrectomy is more cost effective than conservative management (4,5).
Ureteral atresia is a rare congenital anomaly caused by a failure of development of ureteric bud usually associated with a non-functioning kidney. Its combination with another urinary abnormality is even rare (6). The ureter develops from a ureteric bud that grows from the caudal end of the mesonephric duct (Wolffian duct) into the metanephric mesoderm (renal blastema), quickly shifting inferiorly to make its connection with the future bladder. Ureteric bud originates after 28th days of development. The ureter, renal pelvis, calyces and collecting duct of each kidney origin from the ureteric’s bud. During the embryological development alterations in number or position of buds, cause anomalies (bifurcation of renal pelvis or ureter, complete duplication of the system, ectopic ureter). Ureteral atresia derives from a lack of ureter canalization caused by an ischemic injury occurring during the elongation of the ureteral bud, or during the migration of the developing kidney with changes distribution of regional blood supply to the ureter. The ureter may be absent entirely, or it may end blindly after extending only part of the way to the flank. In any event, the result is an atresic ureteral bud and absent or MDK. The MDK is usually unilateral, asymptomatic, of no clinical significance. Rarely, it could be associated with hypertension, infection, or tumor. Contralateral VUR is common, and many clinicians recommend a voiding cystourethrogram as part of the initial workup. The distal atresia is more frequent and is associated with a kidney dysplasia, even if cases of renal function recovery, after removal of the obstruction, are described (7).
By a revision of literature, we have no incidence data about ureteral atresia in each of its possible forms (distal or proximal, complete or partial, short or long). Only 11 cases of ureteral atresia are reported and all describe a distal atresia. Three of these, are related to adult patients (8-10). Between these 11 reports, 4 reported a successful preservation of renal function (Ashimine S, Hedden R, Morozumi M, Macpherson RI) (11-14), while 2 described the association of distal ureteral atresia with ipsilateral ureteropelvic junction obstruction (Bagnara et al. and Shuiqing et al.). Ureteral atresia is often cause of hydronephrosis, due to the obstruction of urine from the kidney. Prenatal diagnosis of hydronephrosis nowadays is possible and, most cases in paediatric patients in fact, are detected by routine ultrasounds screening during pregnancy. The incidence of prenatal hydronephrosis ranges between 0.59% and 1.4% (3). Between the two forms of atresia, the proximal one represents the rarest and we didn’t find any case described.
In our patient, we have a displastic kidney, associated to occasional changes of blood pressure and two episodes of suspected pyelonephritis, with a proximal complete atresia. According us this atresia occurred after the connection between the ureteric bud and the blastema causing an alteration of kidney development; on the contrary, we would have had a renal agenesia. A normal renal function after birth is difficult to explain and to understand in presence of a real atresia of the ureter. In our patient, we didn’t consider the atresia of ureter as mean diagnosis but a MDK associated to a VUR with a chronic reflux damage, cause of infection and possible cause of blood pressure modifications.
In conclusion, since the intraoperative finding and the extreme rarity of this congenital anomaly, as it emerges from a review of the literature, we decided to focus our attention on the atresia of proximal ureter. The causes of ureteral obstruction are various and they must be considered in the classification and in the treatment of this uncommon pathology. A preoperative diagnosis of ureteral atresia is very difficult. Usually in infancy, a distal atresia, presents as an abdominal mass caused by a significant dilatation of the ureter proximally to the atresic segment. If symptomatic, may be present fever, leukocytosis. On the contrary, the atresia of the ureter (usually proximal) with no signs of infection in the dysplastic kidney may be unknown up to adulthood or throughout one’s life. Is possible when the atresia occurs after the pielo-ureteral junction to find a cystic or tubular segment of the ureter, according the side of the atresia, dilated from few to many centimetres presenting as high cystic abdominal mass. There is no better way to diagnose preoperatively an ureteral atresia. Retrograde or anterograde pyelography could be considered the gold standard for diagnosis of the disease when it is suspected. The absence of a segment of the ureter should always be demonstrable to diagnose an atresia. In the reports describing the preservation of renal function, a pyeloplasty or uretero-uretero anastomose was successful. Ureter substitution is also described as possible surgical technique when we have a functioning kidney. In our case, as in other described with a distal atresia, the kidney is involved by a high grade of dysplasia and is not functioning. Nephroureterectomy represent the only possible solution. Retroperitoneoscopic approach allowed us to identify the ureter after the kidney and to visualize correctly and magnify the atresic point ending as cul-de-sac. In our patient the left sided ureter and kidney were normal.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Louw JH, Barnard CN. Congenital intestinal atresia; observations on its origin. Lancet 1955;269:1065-7. [Crossref] [PubMed]
- Sinha RS, Bhattacharjee P, Majhi T. Distal ureteric atresia—A Case Report. J Indian Assoc Pediatr Surg 2002;7:156-8.
- Bagnara V, Castorina S, Nappo SG, et al. Hypothesis on etiopathogenesis, congenital or acquired, of an imperforate distal ureter: a case report. J Med Case Rep 2015;9:227. [Crossref] [PubMed]
- Pérez LM, Naidu SI, Joseph DB. Outcome and cost analysis of operative versus nonoperative management of neonatal multicystic dysplastic kidneys. J Urol 1998;160:1207-11; discussion 1216. [Crossref] [PubMed]
- Bacchetta J, Liutkus A, Dodat H, et al. Multicystic dysplastic kidney disease: update and information for parents at the time of prenatal diagnosis. Arch Pediatr 2008;15:1107-15. [Crossref] [PubMed]
- Wu S, Xu R, Zhu X, et al. Distal ureteral atresia with ureteropelvic junction obstruction in a female child: a rare case. Int J Clin Exp Med 2015;8:1472-4. [PubMed]
- Riccabona M. Obstructive diseases of the urinary tract in children: lessons from the last 15 years. Pediatr Radiol 2010;40:947-55. [Crossref] [PubMed]
- Bhattacharjee PK, Ghosal S, Sharma GD. Distal ureteric atresia presenting as an abdominal lump in an adult. Indian J Surg 2004;66:175-7.
- Karanastasis D, Antoniou N, Tsagatakis E, et al. Distal ureteral atresia associated with ipsilateral renal dysplasia. Scand J Urol Nephrol 1992;26:77-9. [Crossref] [PubMed]
- Floyd MS Jr, Scally J, Irwin PP. Incidental detection of a unilateral dilated blind-ending ureter, renal agenesis, and a dilated seminal vesicle. Urol J 2012;9:639. [PubMed]
- Ashimine S, Miyazato M, Hayashi E, et al. Distal ureteral atresia: recovery of renal function after relief of obstruction at ten months old. Int J Urol 2005;12:578-80. [Crossref] [PubMed]
- Hedden R, Wacksman J, Sheldon C. Complete nonunion of the ureterovesical junction with preservation of renal function. J Urol 1994;151:1361-2. [PubMed]
- Morozumi M, Ogawa Y, Fujime M, et al. Distal ureteral atresia associated with crossed renal ectopia with fusion: recovery of renal function after release of a 10-year ureteral obstruction. Int J Urol 1997;4:512-5. [Crossref] [PubMed]
- Macpherson RI, Gordon L, Bradford BF. Neonatal urinomas: imaging considerations. Pediatr Radiol 1984;14:396-9. [Crossref] [PubMed]


