
Clinical imaging and pathology analysis of Epstein-Barr virus-associated monomorphic lymphoproliferative disease after liver transplantation in children—a retrospective case series
Highlight box
Key findings
• In pediatric patients following liver transplantation, it is essential to implement Epstein-Barr virus (EBV) surveillance, prevention, and early diagnosis of post-transplant lymphoproliferative disease (PTLD), as well as to develop personalized treatment strategies aimed at reducing mortality rates.
What is known and what is new?
• Surveillance for EBV during the initial postoperative year is crucial for the early detection of PTLD. Given the diverse clinical presentations and the nonspecific nature of imaging findings, pathological biopsy is indispensable for achieving an accurate diagnosis.
• The variability in clinical presentations has resulted in an absence of standardized treatment protocols, highlighting the necessity for the exploration of more individualized treatment strategies.
What is the implication, and what should change now?
• The early and definitive diagnosis of PTLD presents significant challenges. It is imperative for clinicians to enhance awareness of this condition, improve monitoring and diagnostic methodologies, and actively investigate personalized treatment strategies.
Introduction
Post-transplant lymphoproliferative disease (PTLD) is the most severe complication following liver transplantation in children. Its increased morbidity and mortality in pediatric patients are closely linked to Epstein-Barr virus (EBV) infection and the use of immunosuppressive medications. According to the 4th edition of the World Health Organization Classification of Lymphoid Neoplasms (WHO-HAEM4R) and the International Consensus Classification of Mature Lymphoid Neoplasms (ICC-2022), PTLD is classified into four subtypes: non-destructive PTLD, polymorphic PTLD, monomorphic PTLD, and classical Hodgkin’s lymphoma PTLD (1,2).
The incidence of PTLD in children is approximately 5.1%, which is notably higher than the 3.8% rate observed in adults, and the time of occurrence is significantly earlier than that in adults, and the development of EBV-positive PTLD lesions occurs significantly earlier than EBV-negative lesions. In addition, nearly half of pediatric PTLDs (49%) occur within 1 year of liver transplantation (3). Compared to polymorphic PTLD, monomorphic PTLD has a poorer prognosis (4).
This research retrospectively examines six cases of EBV-associated monomorphic PTLD arising in the intestines and lymph nodes, delineates the clinical and pathological characteristics of these cases, discusses post-transplantation EBV infections, and proposes evidence-based therapeutic approaches based on existing literature to mitigate the morbidity and mortality associated with PTLD. We present this article in accordance with the AME Case Series reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-311/rc).
Case presentation
This study was a single-center, retrospective, and non-continuous investigation that included a total of six children diagnosed with PTLD. All cases were diagnosed and treated at Chongqing Medical University Affiliated Children’s Hospital, a public institution, between 2018 and 2023. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Chongqing Medical University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from all families of the participating children for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
The demographic information, including age, sex, primary disease, time of onset of PTLD, clinical presentation, imaging manifestations, therapeutic agents utilized, and follow-up results for the six pediatric patients are presented in Table 1 and Figure 1. Additionally, the surveillance data for EBV infection and the results of laboratory liver function tests are detailed in Table 2. The pathological features are illustrated in Table 3 and Figure 2.
Table 1
| Case | Sex/age (months) | Primary disease/surgical approach | Time to PTLD after transplantation | Clinical manifestations | Imaging findings | Immunosuppressive agents used and doses post-transplantation | Therapeutic drugs and programs after the occurrence of PTLD | Prognosis |
|---|---|---|---|---|---|---|---|---|
| 1 | M/16 | Biliary atresia/DDLT | 3 months | Anemia, gastrointestinal bleeding |
Ultrasound: splenomegaly | Tacrolimus capsules: 6 mg, 3/24 h | Acyclovir, Tylenol, vancomycin, cyclosporine A | Died 2 months after diagnosis |
| CT: fluid in the abdominal cavity, multiple pneumoperitonea, and dilatation of the colon and small intestine | Mycophenolate mofetil dispersible tablets: 125 mg, 2/24 h | |||||||
| PET-CT: NA | ||||||||
| 2 | M/95 | Intrahepatic choledocholithiasis/LDLT | 4 months | Fever, anemia, hematochezia, abdominal pain, abdominal distension | Ultrasound: splenomegaly, abdominal effusion | Tacrolimus capsules: 2.5 mg,1/12 h | Acyclovir, Tylenol, cyclosporine A | Died 1 month after diagnosis |
| CT: dilatation of the small bowel with pneumoperitoneum is obvious | Mycophenolate mofetil dispersible tablets:250 mg, 1/12 h | |||||||
| PET-CT: NA | ||||||||
| 3 | M/53 | Portal vein cavernous degeneration/LDLT | 6 months | Blood in stool, anemia, depression |
Ultrasound: splenomegaly | Tacrolimus capsules: 1.5 mg, 1/12 h | Acyclovir, Tylenol, rituximab | Good condition at 13 months follow-up |
| CT: fluid and gas accumulation in the right middle and lower abdominal bowel with dilated bowel lumen | Mycophenolate mofetil dispersible tablets: 250 mg, 1/12 h | |||||||
| PET-CT: NA | ||||||||
| 4 | F/30 | Biliary atresia/LDLT | 2 years | Anemia, vomiting, abdominal mass | Ultrasound: splenomegaly | Tacrolimus capsules: 0.833 mg, 1/12 h | Acyclovir, rituximab + COPADM3/CVM | Good condition at 6 months follow-up |
| CT: right abdominal space-occupying lesion with a close relationship to the bowel | Mycophenolate mofetil dispersible tablets: 125 mg, 1/12 h | |||||||
| PET-CT: intestinal lymphoma with multiple groups of enlarged lymph nodes in the mesentery and abdominal aorta | ||||||||
| 5 | F/41 | Biliary atresia/LDLT | 3 years | Anemia, vomiting, bloating, convulsions | Ultrasound: intestinal wall thickening and intestinal pneumatization | Tacrolimus capsules: 0.75 mg, 1/24 h | Acyclovir, BFM-95 regimen, V + Ar + BBr | Good condition at 6 months follow-up |
| CT: thickening of intestinal wall with narrowing of intestinal lumen | Mycophenolate mofetil dispersible tablets: 125 mg, 1/12 h | Chemotherapy | ||||||
| PET-CT: NA | ||||||||
| 6 | F/62 | Progressive familial intrahepatic cholestasis/LDLT | 10 months | Anemia, abdominal pain, fever, generalized lymphadenopathy | Ultrasound: hepatosplenomegaly, pleural and abdominal effusion | Tacrolimus capsules: 1.5 mg, 1/12 h | Modified CHOP | Died 3 months after diagnosis |
| CT: bilateral cervical and right axillary lymph node enlargement, Pleural effusion and hilar lymphadenopathy | Mycophenolate mofetil dispersible tablets: 250 mg, 1/12 h | Chemotherapy, acyclovir, Tylenol | ||||||
| PET-CT: diffuse lymphadenopathy with splenomegaly |
M, male; F, female; DDLT, deceased donor liver transplantation; LDLT, related living donor liver transplantation; PTLD, post-transplant lymphoproliferative disease; CT, computed tomography; PET, positron emission tomography; NA, not done; COPADM3/CVM, C, cyclophosphamide O, vincristine; P, dexamethasone; A, doxorubicin; D, cytarabine; M, methotrexate; C, cyclophosphamide; V, vincristine; M, methotrexate; BFM-95, Berlin-Frankfurt-Münster 95; V + Ar + BBr, vinorelbine + doxorubicin + bleomycin; CHOP, cyclophosphamide + doxorubicin hydrochloride + vincristine sulfate + prednisone.

Table 2
| Case | Pre-transplant donor serum EBV status | Pre-transplant recipient serum EBV status | Serum EBV-PCR (copies/mL) post-transplantation | Receptor serum EBV status at PTLD | Serum EBV-PCR at PTLD (copies/mL) | HB (g/L) | ALB (g/L) | ALT (U/L) | AST (U/L) | PT (seconds) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NA | NA | NA | CA-IgG, NA-IgG | NA | 66 ↓ | 25.5 ↓ | 69.2 ↑ | 42 | 17.6 |
| 2 | NA | NA | NA | CA-IgG | NA | 104 ↓ | 30.6 ↓ | 12.2 | 14.2 | 18.2 |
| 3 | NA | EBV-PCR 7.25×103 copies/mL | <400 | CA-IgG | 2.68×104 | 107 ↓ | 32.2 ↓ | 12 | 54 ↑ | 22.5 ↑ |
| 4 | NA | EBV-PCR <400 copies/mL | <400 | CA-IgG, EA-IgG, NA-IgG | 4.68×106 | 92 ↓ | 29.4 ↓ | 9 | 35 | 17.4 |
| 5 | CA-IgG | EBV-PCR <400 copies/mL | <400 | CA-IgG, EA-IgG, NA-IgG | 4.31×104 | 48 ↓ | 51.4 | 29 | 20 | 22.6 ↑ |
| 6 | NA | EBV-PCR <400 copies/mL | 3.52×103 | CA-IgG | 9.8×104 | 79 ↓ | 35.7 ↓ | 13 | 29 | 16.9 |
↓: elevation; ↑: reduction. PTLD, post-transplant lymphoproliferative disorder; EBV, Epstein-Barr virus; PCR, polymerase chain reaction; NA, not done; CA-IgG, capsid antigen immunoglobulin G; NA-IgG, nuclear antigen immunoglobulin G; EA-IgG, early antigen immunoglobulin G; HB, hemoglobin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PT, plasminogen time.
Table 3
| Case | Sites and biopsy modalities | Pathological type | EBERs ISH | EBNA2 | CD20 | CD3 | BCL2 | BCL6 | CD10 | C-MYC | CD30 | PD-1 | PD-L1 | P53 | Ki67 | Detection of clonal gene rearrangements |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jejunum/surgical resection | DLBCL | + | + | + | 10%+ | − | − | − | − | + | − | + | 20% | 40%+ | IgH, Igk |
| 2 | Ileum/surgical resection | DLBCL | + | + | + | 10%+ | − | − | − | − | + | − | + | 20% | 60%+ | IgH, Igk, TCRγ |
| 3 | Jejunum/surgical resection | DLBCL | + | + | + | 40%+ | − | − | − | − | + | − | + | 30% | 30%+ | IgH, Igk |
| 4 | Colonic hepatic area/puncture biopsy | Burkitt | 20%+ | − | + | − | − | + | + | + | − | − | 40%+ | 40% | >95%+ | NA |
| 5 | Jejunum/surgical resection | Burkitt | 20%+ | − | + | − | − | + | + | + | − | − | + | 80% | >95%+ | NA |
| 6 | Cervical lymph nodes/surgical removal | PTCL-NOS | 40%+ | − | − | + | − | − | − | − | − | 10%+ | 50%+ | 10% | 40%+ | TCRγ |
Note: The target of observation was the tumor cells. PTLD, post-transplant lymphoproliferative disorder; EBERs ISH, Epstein-Barr virus-encoded RNA in situ hybridization; DLBCL, diffuse large B-cell lymphoma; Burkitt, Burkitt lymphoma; PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; NA, not done; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; Ig, immunoglobulin.
Cases 1 to 5 of PTLD were all localized within the gastrointestinal tract, resulting in a relatively uniform clinical presentation. Each patient was admitted to the hospital due to gastrointestinal complications, which included symptoms such as anemia, hematochezia, abdominal distension, abdominal pain, or the presence of an abdominal mass. Imaging studies indicated that cases 1, 2, and 3 predominantly exhibited intestinal pneumoperitoneum, intestinal lumen dilation, abdominal effusion, and splenomegaly (see Figure 1A). In contrast, case 4 presented with a mass in close proximity to the bowel (see Figure 1C), while case 5 suggested bowel wall thickening and narrowing of the bowel lumen (see Figure 1B). Laboratory analyses revealed varying degrees of anemia and impaired liver function across all five cases. EBV testing indicated that all five children were negative for EBV prior to liver transplantation; however, at the time of PTLD diagnosis, both serum antibody testing and EBV-DNA testing suggested the presence of EBV infection (refer to Table 2).
During the hospitalization and diagnostic process, cases 1 and 2 presented with severe conditions characterized by intestinal perforation, peritoneal effusion, and systemic infection. Both cases underwent partial intestinal resection, intestinal repair, intestinal mucosal laxation, and abdominal drainage. Following the clarification of their diagnoses, immunosuppressive medications were discontinued, and the patients received anti-infective and supportive treatments. However, the infection could not be effectively controlled and subsequently progressed to pneumonia, ultimately resulting in death due to infectious shock and respiratory failure.
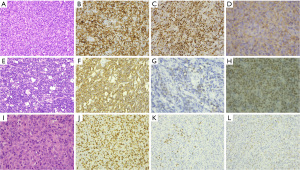
Cases 3, 4, and 5 exhibited improved baseline conditions following the surgical resection of the intestinal lesion. After the diagnosis of PTLD was established, the immunosuppressive therapy was reduced and subsequently discontinued. These patients received anti-inflammatory and anti-neoplastic treatments, including rituximab and combination chemotherapy, and demonstrated favorable outcomes at the follow-up visits conducted 6 to 13 months post-treatment. Pathological examinations of cases 1, 2, and 3 confirmed a diagnosis of diffuse large B-cell type PTLD (refer to Figure 2A-2D, Table 3), while cases 4 and 5 were diagnosed with Burkitt lymphoma type PTLD (refer to Figure 2E-2H, Table 3).
In contrast to the preceding five cases, the clinical presentation of case 6 was characterized primarily by generalized lymphadenopathy. Imaging studies indicated the presence of generalized lymph node enlargement, hilar lymphadenopathy, and pleural effusion (refer to Figure 1D). Additionally, ultrasound and positron emission tomography (PET)-computed tomography (CT) findings suggested splenomegaly and intestinal pneumoperitoneum. Pathological examination confirmed the diagnosis of T-cell type PTLD [peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS)] (see Figure 2I-2L, Table 3). Following the diagnosis, the patient received antiviral and anti-infective therapy; however, they subsequently developed secondary hemophagocytic syndrome, bone marrow suppression, and severe infection, ultimately resulting in death three months after the diagnosis.
Discussion
The risk of PTLD occurrence is associated with various factors such as the recipient’s age, location of the disease, EBV infection status of the donor and recipient pre- and post-transplantation, presence of concurrent viral infections, intensity and duration of immunosuppression, and time elapsed since the transplantation (5). Notably, EBV infection plays a crucial role in the pathogenesis of PTLD (6). In this investigation, the transplant cases had negative serum EBV antibody tests before the transplantation procedure. However, upon the onset of PTLD, all cases tested positive for the EBV test, indicating a strong correlation between EBV infection and PTLD.
Due to the decline in both the number and function of T cells in recipients as a result of immunosuppressive drug therapy, EBV-infected B cells cannot be effectively controlled by EBV-specific cytotoxic T cells. The unchecked proliferation of these cells may ultimately lead to the development of PTLD (7). EBV upregulates the programmed cell death protein 1 (PD-1) inhibitory receptor and its ligand programmed cell death ligand 1 (PD-L1) on the surface of T cells, resulting in the dysfunction of effector cells becoming unable to control infection (8).
Consequently, vigilant surveillance of EBV infection following transplantation can serve as a crucial indicator for clinicians regarding the potential development of PTLD, particularly in instances characterized by lymphadenopathy. This article presents four cases in which monthly postoperative EBV monitoring was conducted, with adjustments made to the immunosuppressant regimen based on the monitoring outcomes. However, in the initial cases (1 and 2), EBV monitoring was not performed postoperatively due to specific circumstances, resulting in the detection of EBV infection only upon the patients’ readmission for PTLD, which had already progressed to a severe and poorly managed state.
The majority of the six cases examined in this study exhibited gastrointestinal lesions, which may be attributed to the substantial presence of lymphoid tissue in this anatomical region. Reiche (9) conducted a statistical analysis of nine studies on PTLD and found that the gastrointestinal tract was the organ most frequently affected, with specific involvement of the colorectum (18%), jejunum (12%), gastroduodenum (9%), and esophagus (3%), in that order (10). Among the cases of PTLD occurring in the gastrointestinal tract discussed in this paper, 4 out of 5 were located in the small intestine, while only one case was identified in the colon. Further statistical analysis of additional cases is warranted.
The clinical manifestations of patients with PTLD exhibit considerable variability depending on the cumulative sites involved and lack specificity. A definitive diagnosis necessitates a pathological examination. The fifth edition of the World Health Organization Classification of Lymphohaematopoietic Tumours (WHO-HAEM5) has introduced significant modifications to the classification of lymphomas associated with immunodeficiency or immunocompromise, notably the removal of the classification for post-transplant PTLD. The revised nomenclature comprises three primary components: histopathological diagnosis, the presence of oncogenic viral infection, and the clinical or immunodeficiency background. This underscores the necessity for a comprehensive diagnostic approach that integrates both pathological and clinical factors (11). However, the 2022 edition of the International Consensus Classification of Haemato-Oncology (ICC-2022) acknowledges that the clinical management of PTLD following organ transplantation is distinctly different from that of immunodeficient PTLD arising from other etiologies. Consequently, its classification continues to rely on the criteria established in the WHO-HAEM4R.
According to the classification methodology employed in this study, all cases examined were identified as monomorphic PTLD. Among these, three cases were classified as diffuse large B-cell lymphoma [DLBCL, non-germinal center B-cell (non-GCB) type], two as Burkitt lymphoma, and one as peripheral T-cell lymphoma (PTCL-NOS). Monomorphic PTLD constitutes approximately 60–80% of all PTLD cases, with over 80% originating from B-cells. DLBCL is the most prevalent subtype, accounting for approximately 60% of cases, followed by Burkitt lymphoma and T-cell PTLD. Furthermore, EBV-positive B-cell PTLD is frequently associated with T-cell TCRγ rearrangements, which are typically oligoclonal or limited, indicating a potential impairment in immune surveillance due to T-cell attenuation (12,13).
Morphological subtypes play a crucial role in guiding the selection of specific treatments and assessing prognosis. In this study, case 6 was classified as T-cell PTLD. T-cell PTLD is regarded as a rare condition, representing approximately 10–15% of all cases, and is characterized by a significant degree of heterogeneity, with PTCL-NOS being the most prevalent histological subtype (14). Typically, T-cell PTLD exhibits a prolonged interval between engraftment and diagnosis, and is accompanied by EBV infection in approximately one-third of cases, which is linked to a poor prognosis (4,15).
The prognosis of Burkitt-type PTLD was more favorable compared to DLBCL-type PTLD, with the patient still alive at the 6-month follow-up. Notably, in the case of DLBCL-type PTLD, the presence of more T lymphocytes in the tumor tissue and morphological consistency with T-rich DLBCL features indicated a potentially better prognosis, as evidenced by the patient’s good condition at the 13-month follow-up (16).
There exists a substantial correlation between the administration of immunosuppressive agents and the annual rise in the prevalence of monotypic PTLD. This phenomenon is likely attributable to the fact that tacrolimus extends the lifespan of transplanted patients while concurrently heightening their vulnerability to EBV (17,18). The primary therapeutic strategy involves either reducing or discontinuing the use of immunosuppressive medications; however, the optimal timing for the resumption of immunosuppression following PTLD remission, as well as the appropriate dosage, remains ambiguous. This uncertainty underscores the necessity for a more individualized treatment approach (19,20).
In pediatric patients, the recommended first-line chemotherapy regimen in combination with rituximab is rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone (21). Cases 1 and 2 did not undergo EBV surveillance due to familial circumstances, experienced rapid disease progression following the onset of their condition, lacked the necessary criteria to receive rituximab combination chemotherapy, and consequently exhibited a poor prognosis. In contrast, cases 3, 4, and 5 received both EBV surveillance and rituximab combination chemotherapy, resulting in a more favorable prognosis.
A variety of therapeutic approaches are currently under investigation, including EBV-specific cytotoxic T-lymphocyte (CTL) immunotherapy. This method involves the utilization of autologous or third-party-derived EBV-specific CTLs to target and eliminate aberrant B-cells, thereby reducing EBV DNA load and significantly enhancing remission rates as well as 3-year survival outcomes in refractory PTLD cases (22,23). Additionally, for patients with B-cell PTLD that express CD30, the anti-CD30 agent brentuximab vedotin (BV) is available and has demonstrated potential efficacy in individuals with PTLD who have not responded to initial therapies, as well as in cases of T-cell PTLD characterized by a poor prognosis (24).
PD-L1 expression is a prevalent characteristic of EBV-associated lymphoproliferative disorders (LPD) and indicates potential immune evasion. Monomorphic PTLD exhibits elevated PD-L1 expression and reduced PD-1 expression compared with polymorphic PTLD (25). Consistent with the existing literature, our case demonstrated heightened PD-L1 expression in tumor cells, particularly in cases of DLBCL with minimal PD-1 expression. Consequently, PD-L1 immunotherapy may hold promise for the treatment of monomorphic PTLD.
This is a single-center, retrospective study involving a small sample of pediatric patients. The postoperative observation period ranged from 6 to 13 months, and continued follow-up is necessary to determine long-term prognosis and recurrence rates. Clinical findings and therapeutic medications were extracted from medical records; however, there was no control group to evaluate potential biases in the results due to errors in exclusion documentation. Therefore, prospective or controlled studies with a larger, multicenter patient population are needed to gather more comprehensive clinical data.
Conclusions
Monomorphic PTLD is associated with a high mortality rate and presents with a complex clinicopathological profile. Consequently, it is imperative to conduct early monitoring of serum EBV levels, identify high-risk patients based on their clinical presentation, facilitate prompt diagnosis, and develop individualized treatment regimens. These measures are essential for enhancing the overall survival rates of pediatric patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-311/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-311/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-311/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of Chongqing Medical University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from all families of the participating children for publication of this case series and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375-90. [PubMed]
- Campo E, Jaffe ES, Cook JR, et al. The International Consensus Classification of Mature Lymphoid Neoplasms: a report from the Clinical Advisory Committee. Blood 2022;140:1229-53. [PubMed]
- Tajima T, Hata K, Haga H, et al. Post-transplant Lymphoproliferative Disorders After Liver Transplantation: A Retrospective Cohort Study Including 1954 Transplants. Liver Transpl 2021;27:1165-80. [PubMed]
- Koff JL, Li JX, Zhang X, et al. Impact of the posttransplant lymphoproliferative disorder subtype on survival. Cancer 2018;124:2327-2336. [PubMed]
- Marie E, Navallas M, Navarro OM, et al. Posttransplant Lymphoproliferative Disorder in Children: A 360-degree Perspective. Radiographics 2020;40:241-65. [PubMed]
- Crombie JL, LaCasce AS. Epstein Barr Virus Associated B-Cell Lymphomas and Iatrogenic Lymphoproliferative Disorders. Front Oncol 2019;9:109. [PubMed]
- Stojanova J, Caillard S, Rousseau A, et al. Post-transplant lymphoproliferative disease (PTLD): Pharmacological, virological and other determinants. Pharmacol Res 2011;63:1-7. [PubMed]
- DeStefano CB, Desai SH, Shenoy AG, et al. Management of post-transplant lymphoproliferative disorders. Br J Haematol 2018;182:330-43. [PubMed]
- Reiche W, Tauseef A, Sabri A, et al. Gastrointestinal manifestations, risk factors, and management in patients with post-transplant lymphoproliferative disorder: A systematic review. World J Transplant 2022;12:268-80. [PubMed]
- Dziegielewski C, Contreras R, Weitzman S, et al. Pediatric Gastrointestinal Posttransplant Lymphoproliferative Disorder: Incidence, Clinical Characteristics, and Impact of Major Surgical Interventions Upon Overall Survival. J Pediatr Hematol Oncol 2018;40:438-44. [PubMed]
- Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022;36:1720-48.
- Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood 2011;117:4726-35. [PubMed]
- Swerdlow SH, Chadburn A, Ferry JA, et al. Post-transplant lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al. editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (revised 4th ed.). Lyon, France: IARC; 2017:453-62.
- Tiede C, Maecker-Kolhoff B, Klein C, et al. Risk factors and prognosis in T-cell posttransplantation lymphoproliferative diseases: reevaluation of 163 cases. Transplantation 2013;95:479-88. [PubMed]
- Jiang C, Huang J, Shao J, et al. T-Cell Posttransplant Lymphoproliferative Disorders After Allogeneic Hematopoietic Stem Cell Transplantation: Case Series and Systemic Review. Cell Transplant 2024;33:9636897241259722. [PubMed]
- Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood 2015;126:863-72. [PubMed]
- Tsai DE, Bagley S, Reshef R, et al. The changing face of adult posttransplant lymphoproliferative disorder: Changes in histology between 1999 and 2013. Am J Hematol 2018;93:874-81. [PubMed]
- Fukushima D, Sato K, Kawagishi N, et al. Epstein-Barr virus--associated posttransplantation lymphoproliferative disorder with tacrolimus metabolism deterioration in infants after living-donor liver transplantation. Transplantation 2015;99:114-9. [PubMed]
- Okamoto T, Okajima H, Uebayashi EY, et al. Management of Epstein-Barr Virus Infection and Post-Transplant Lymphoproliferative Disorder in Pediatric Liver Transplantation. J Clin Med 2022;11:2166. [PubMed]
- Liu Y, Wang BC, Zuppan CW, et al. Relationship of Post-Transplant Lymphoproliferative Disorders (PTLD) Subtypes and Clinical Outcome in Pediatric Heart Transplant Recipients: A Retrospective Single Institutional Analysis/Experience of 558 Patients. Cancers (Basel) 2023;15:976. [PubMed]
- Janeela AM, Fouzia NA, Zachariah UG. Post-transplantation Lymphoproliferative Disorder (PTLD): In the Liver Transplant Recipient. J Clin Exp Hepatol 2024;14:101286. [PubMed]
- Liu JY, Zhang JM, Zhan HS, et al. EBV-specific cytotoxic T lymphocytes for refractory EBV-associated post-transplant lymphoproliferative disorder in solid organ transplant recipients: a systematic review. Transpl Int. 2021;34:2483-93. [PubMed]
- Kazi S, Mathur A, Wilkie G, et al. Long-term follow up after third-party viral-specific cytotoxic lymphocytes for immunosuppression- and Epstein-Barr virus-associated lymphoproliferative disease. Haematologica 2019;104:e356-9. [PubMed]
- Hong J, Johnson WT, Kartan S, et al. Durable Response to Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, and Prednisone (BV-CHP) in a Patient with CD30-Positive PTCL Arising as a Post-Transplant Lymphoproliferative Disorder (PTLD). Curr Oncol 2021;28:5067-72. [PubMed]
- Schiefer AI, Salzer E, Füreder A, et al. PD-L1 and PD1 expression in post-transplantation lymphoproliferative disease (PTLD) of childhood and adolescence: An inter- and intra-individual descriptive study covering the whole spectrum of PTLD categories. Cancer Med 2019;8:4656-68. [PubMed]


