
Role of systemic inflammation response index and prognostic nutritional index in the prediction of moderate-to-severe bronchopulmonary dysplasia in very preterm infants
Highlight box
Key findings
• This study focused on evaluating the predictive and diagnostic ability of systemic inflammation indices [systemic inflammation response index (SIRI), prognostic nutritional index (PNI)] for bronchopulmonary dysplasia (BPD) in premature infants.
What is known and what is new?
• Inflammatory cascade is an important mechanism for the occurrence of BPD. This process involves a variety of biomarkers as markers for early prediction of the occurrence and prognosis of BPD.
• The contribution of PNI to diagnosing BPD is not established. The study aimed to investigate the potential clinical value of PNI in predicting moderate-to-severe BPD.
What is the implication, and what should change now?
• Within 24 hours after birth and 36 weeks of postmenstrual age, systemic inflammation indices such as SIRI and PNI of preterm infants with BPD have specific predictive and diagnostic values.
IntroductionOther Section
Bronchopulmonary dysplasia (BPD) refers to the need for oxygen or respiratory support at 36 weeks of postmenstrual age (PMA). BPD is not only the most common complication of premature infants (1), but also an important risk factor for other complications of premature infants and a variety of chronic non-communicable diseases (2). However, despite significant advancements in treatment and management for patients with BPD (3), its incidence remains high. The prognosis for premature infants with varying grades of BPD differs, with more severe BPD associated with a higher risk of adverse respiratory and neurological outcomes (4,5). Accurate assessment of BPD severity is essential for the identification of high-risk infants and the implementation of appropriate interventions. Nevertheless, in clinical practice, evaluating the severity of graded BPD at 36 weeks of PMA or at discharge, potentially overlooking the opportunity for early and effective treatment. Therefore, exploration of biomarkers for early prediction of BPD in preterm infants is the current focus and challenge of research.
Inflammatory injury, oxidative stress, immune imbalance, and other mechanisms are involved in the occurrence and progression of BPD. Among them, persistent inflammatory response leads to immature lung injury and abnormal development of lung tissue and pulmonary vessels, which is an important mechanism of BPD (6-8). A variety of biomarkers are involved in the process of BPD. Studies have found that there are a variety of biomarkers in placenta, umbilical cord blood, and body fluids of BPD patients, including various types of cells, cytokines, lipids, proteins, and microRNA (9-11). These markers can be detected within a few weeks or even hours after birth in premature infants with BPD, which is helpful for early prediction, diagnosis, and prognosis evaluation of BPD. However, most biomarkers cannot be widely used and promoted in clinical practice, and the relationship with BPD risk is still uncertain. Hematological examination is a routine clinical operation to evaluate the condition of premature infants in different periods, including at birth. Different types of blood cells play an important role in lung inflammation and are related to lung injury in preterm infants (12). Prior research has assessed the predictive and diagnostic significance of systemic inflammatory indices [such as neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammation index (SII)] in BPD (13,14). The variability in findings regarding BPD and systemic inflammatory indices in previous research necessitates exploring alternative inflammatory markers for predicting and diagnosing BPD. The systemic inflammation response index (SIRI) and prognostic nutritional index (PNI) serve as comprehensive indicators of immune status and are linked to various diseases (15,16). The association between systemic inflammatory indices (SIRI, PNI) and the risk of BPD in preterm infants remains unclear. This study mainly evaluated the systemic inflammatory indices (SIRI, PNI) in the early postnatal period and at 36 weeks of PMA, aiming identify potential biomarkers for predicting and diagnosing BPD. We present this article in accordance with the TRIPOD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-381/rc).
MethodsOther Section
This was a retrospective study, carried out in The First Affiliated Hospital of Xinjiang Medical University Neonatal Intensive Care Unit between January 2017 and December 2022. The study included premature infants with a gestational age (GA) of less than 32 weeks. The Institutional Review Board (IRB) of The First Affiliated Hospital of Xinjiang Medical University granted ethical approval (No. K202212-12). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Demographic features and clinical outcomes
Data collected for all eligible patients included GA, birth weight (BW), gender, antenatal steroid treatment, cesarean delivery, antepartum infection, 1st and 5th minute Apgar scores, duration of mechanical ventilation, early onset sepsis (EOS), late onset sepsis (LOS), grade III–IV intraventricular hemorrhage (IVH), hemodynamically significant patent ductus arteriosus (hsPDA), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), and duration of intravenous nutrition.
Definition of BPD
BPD was defined based on neonatal research network (NRN) data from Jensen et al.’s study (17). BPD severity is categorized by the respiratory support required at 36 weeks of PMA, excluding any previous or ongoing oxygen therapy. No BPD is defined as requiring no respiratory support; Grade 1 BPD involves using a nasal cannula with flow rates ≤2 L/min; Grade 2 BPD includes the use of a nasal cannula with flow rates >2 L/min or noninvasive positive airway pressure; Grade 3 BPD is characterized by the need for invasive mechanical ventilation.
Grade 2–3 BPD was defined as moderate-to-severe BPD; infants with moderate-to-severe BPD (msBPD) were included in the msBPD group. Infants with non-BPD and grade 1 BPD were included in the control group.
Blood analysis
Blood samples from peripheral veins were collected from all preterm infants within 24 hours after birth (24 h) and at the 36th week of PMA. Neutrophil count (109/L) (NEUT), lymphocyte count (109/L) (LYM), monocyte count (109/L) (MONO), platelet count (1012/L) (PLT), and albumin (g/L) (ALB) values were recorded. The systemic inflammatory indexes were formulated using the following methods. SIRI = N × M/L, and PNI = A +5 × L.
Statistical analysis
The software SPSS 22.0 (IBM Corp., Armonk, NY, USA) was used to analyze the data. Numerical variables were expressed as the mean ± standard deviation (x ± SD) or median and interquartile range (IQR). Frequency was the method of representation for categorical variables. For statistical analysis, either Fisher’s exact test or Pearson chi-squared test was used for categorical data, whereas continuous data was analyzed with the t-test or Mann-Whitney U test. The receiver operating characteristic (ROC) curve was drawn to analyze the value of SIRI and PNI in predicting msBPD, and the optimal cut-off point was determined. A P value of <0.05 was considered statistically significant.
ResultsOther Section
During the study period, a total of 491 infants were included in the study. There were 435 premature infants included in the control group and 56 premature infants included in the msBPD group. Gender (P=0.95), cesarean section (P=0.08), antepartum infection (P=0.80), and NEC (P=0.41) were found to be similar between the two groups. GA and BW were significantly lower in the msBPD group (P<0.001, respectively). The msBPD group exhibited lower Apgar scores at both 1 and 5 minutes when compared to the control group (P=0.02 and P=0.04, respectively). The durations of mechanical ventilation and intravenous nutrition were significantly longer in the msBPD group (P<0.001, respectively). Compared with the control group, the msBPD group had higher rates of IVH, hsPDA, ROP (P<0.001, respectively), EOS (P=0.049), and LOS (P=0.007) (Table 1).
Table 1
| Characteristics | Control group (N=435) | msBPD group (N=56) | P value |
|---|---|---|---|
| GA, weeks | 29.80±1.18 | 27.83±1.80 | <0.001 |
| BW, g | 1,333.61±266.90 | 1,019.54±222.57 | <0.001 |
| Male | 235 (54.02) | 30 (53.57) | 0.95 |
| Cesarean section | 313 (71.95) | 34 (60.71) | 0.08 |
| Antepartum infection | 27 (6.21) | 3 (5.36) | 0.80 |
| Antenatal steroid | 204 (46.90) | 16 (28.57) | 0.009 |
| Apgar 1st min | 7.21±1.87 | 6.59±1.62 | 0.02 |
| Apgar 5th min | 8.85±1.03 | 8.55±0.83 | 0.04 |
| Duration of MV, days | 0.00 (0.00, 6.00) | 23.00 (13.00, 30.00) | <0.001 |
| Duration of intravenous nutrition, days | 23.00 (18.00, 30.00) | 39.00 (29.00, 51.50) | <0.001 |
| NEC | 53 (12.18) | 9 (16.07) | 0.41 |
| ROP | 47 (10.80) | 19 (33.93) | <0.001 |
| hsPDA | 63 (14.48) | 22 (39.29) | <0.001 |
| EOS | 144 (33.10) | 26 (46.43) | 0.049 |
| LOS | 52 (11.95) | 14 (25.00) | 0.007 |
| IVH | 138 (31.72) | 40 (71.43) | <0.001 |
Data are presented as mean ± standard deviation, n (%), or median (interquartile range). msBPD, moderate-to-severe bronchopulmonary dysplasia; GA, gestational age; BW, birth weight; MV, mechanical ventilation; NEC, necrotizing enterocolitis; ROP, retinopathy of prematurity; hsPDA, hemodynamically significant patent ductus arteriosus; EOS, early onset sepsis; LOS, late onset sepsis; IVH, intraventricular hemorrhage.
N (P=0.14), L (P=0.56), and PLTs (P=0.22) at 24 hours were similar between the two groups. NEUT (P=0.52), M (P=0.22), and ALB values (P=0.05) at 36 weeks of PMA were similar between the two groups. In the blood obtained at 24 hours, MONO count (median: 1.15, P=0.04) and SIRI value (median: 2.13, P=0.02) in the msBDP group were higher than those in the control group; albumin value (median: 26.31, P=0.002) and PNI value (median: 36.59, P=0.03) in the msBDP group were lower than those in the control group. In the blood obtained at 36 weeks of PMA, SIRI values (median: 3.80, P=0.01) in the msBDP group were higher than those in the control group, whereas LYM count (median: 2.75, P=0.001), PLTs (median: 161, P<0.001), and PNI values (median: 42.55, P<0.001) in the msBDP group were lower than in the control group (Table 2).
Table 2
| Time | Characteristics | Control group (N=435) | msBPD group (N=56) | P value |
|---|---|---|---|---|
| Within 24 hours after birth | Neutrophil count (109/L) | 3.22 (1.81, 5.62) | 4.04 (2.12, 7.45) | 0.14 |
| Lymphocyte count (109/L) | 2.16 (1.58, 3.02) | 2.08 (1.62, 2.83) | 0.56 | |
| Monocyte count (109/L) | 0.92 (0.60, 1.46) | 1.15 (0.57, 2.44) | 0.04 | |
| Platelet count (1012/L) | 201.00 (149.00, 249.50) | 178.50 (139.75, 222.75) | 0.22 | |
| Albumin (g/L) | 27.70 (25.70, 29.78) | 26.31 (23.53, 28.45) | 0.002 | |
| SIRI | 1.30 (0.66, 2.99) | 2.13 (0.80, 5.05) | 0.02 | |
| PNI | 38.97 (35.05, 43.46) | 36.59 (32.22, 42.55) | 0.03 | |
| 36th week PMA | Neutrophil count (109/L) | 4.73 (3.08, 7.35) | 4.99 (2.89, 9.73) | 0.52 |
| Lymphocyte count (109/L) | 3.67 (2.80, 4.54) | 2.75 (2.27, 4.06) | 0.001 | |
| Monocyte count (109/L) | 1.85 (1.39, 2.50) | 2.13 (1.34, 2.82) | 0.22 | |
| Platelet count (1012/L) | 221.00 (152.00, 294.00) | 161.00 (109.75, 222.25) | <0.001 | |
| Albumin (g/L) | 29.21 (26.92, 31.69) | 28.30 (24.75, 30.54) | 0.05 | |
| SIRI | 2.39 (1.30, 4.55) | 3.80 (1.57, 7.71) | 0.01 | |
| PNI | 47.69 (41.59, 53.02) | 42.55 (38.70, 48.08) | <0.001 |
Data are presented as median (interquartile range). PMA, postmenstrual age; msBPD, moderate-to-severe bronchopulmonary dysplasia; SIRI, systemic inflammation response index; PNI, prognostic nutritional index.
To determine the statistical significance of SIRI and PNI, ROC analysis was performed, indicating significance for msBPD diagnosis at 24 hours and at 36 weeks of PMA.
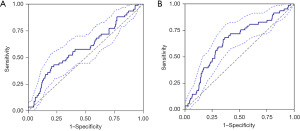
At 24 hours, the area under the curve (AUC) for SIRI in predicting msBPD was 0.599 [95% confidence interval (CI): 0.514–0.685], with a cut-off value >1.927. At the 36th week of PMA, the AUC for diagnosing msBPD was 0.602 (95% CI: 0.515–0.689), with a cut-off value >5.175. The AUC value of PNI for msBPD predictivity at 24 hours was 0.588 (95% CI: 0.504–0.672) and the diagnostic cut-off value was <34.105. For the diagnosis of msBPD at 36 weeks of PMA, the AUC value of PNI was 0.647 (95% CI: 0.569–0.725) and the cut-off value was <45.080. The results of ROC analysis are presented Table 3 and Figures 1,2.
Table 3
| Parameters | AUC (95% CI) | Cut-off | SEN | SPE | PPV | NPV |
|---|---|---|---|---|---|---|
| SIRI within 24 hours after birth | 0.599 (0.514–0.685) | >1.927 | 0.571 | 0.653 | 0.175 | 0.922 |
| SIRI at 36 weeks of PMA | 0.602 (0.515–0.689) | >5.175 | 0.446 | 0.800 | 0.223 | 0.918 |
| PNI within 24 hours after birth | 0.588 (0.504–0.672) | <34.105 | 0.411 | 0.798 | 0.207 | 0.913 |
| PNI at 36 weeks of PMA | 0.647 (0.569–0.725) | <45.080 | 0.679 | 0.634 | 0.193 | 0.939 |
SIRI, systemic inflammation response index; PNI, prognostic nutritional index; ROC, receiver operating characteristic; PMA, postmenstrual age; AUC, area under the curve; CI, confidence interval; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value.

DiscussionOther Section
With the progress of perinatal comprehensive management technology, the survival rate of very premature infants has been significantly improved, and other complications except BPD have shown a downward trend. However, the incidence of BPD is still increasing, with moderate-to-severe BPD showing a significantly high prevalence (18). Compared with mild BPD, msBPD seriously affects the quality of life of surviving patients, causing a huge burden to families and society (2,19). There remain substantial gaps in the best practices and optimal management of msBPD. Hence, early prediction of msBPD is crucial in preterm infants. Early diagnosis of BPD is a hot spot in clinical research by discovering possible markers of BPD. This study evaluated the role of SIRI and PNI in the predictivity of msBPD. It was found that SIRI value >1.927 and PNI value <34.105 within 24 hours at birth were effective biomarkers in the prediction of probable msBPD. The combination of SIRI value >5.175 and PNI <45.080 at 36 weeks of PMA was shown to be a parameter for the diagnosis of msBPD.
Due to immature development, premature infants lack immunoglobulins and antimicrobial peptides, the total number and maturity of immune cells is insufficient, and the level of pro-inflammatory cytokines is increased. Following exposure to inflammatory stimuli and subsequent host innate immune responses, the regulation of related inflammatory cells and inflammatory mediators is abnormal, the initiation of the inflammatory cascade in immature lung tissue interferes with normal interval formation and alveolar development, leading to the typical characteristics of alveolar reduction and enlargement, pulmonary vascular malformation, and alveolar damage, and subsequently the occurrence and progression of BPD (20,21). Overall, the imbalance between pro-inflammatory and anti-inflammatory factors leads to immune imbalance, contributing to an increased risk of BPD, which is clinically manifested as abnormal hematological parameters. The pathogenesis of BPD primarily involves immune cells such as NEUT, MONO, and LYM (9). PLT play an important role in inflammatory response, immune response, oxidative stress, and angiogenesis, and participates in the occurrence and progression of BPD by regulating the expression of cytokines such as vascular endothelial growth factor and platelet-derived growth factor (22-24). ALB is an important indicator reflecting the nutritional and inflammatory status of premature infants. Studies have shown that serum ALB not only maintains the colloid osmotic pressure and endothelial stability of the body, but also plays an important role in anti-oxidation, anti-inflammation, and improving endothelial injury (24,25). Research indicates a correlation between ALB levels and both the onset and outcome of neonatal infections and adult acute respiratory distress (24). Due to the inconsistent findings regarding BPD and the numerical variation of immune cells across various studies, it is essential to explore additional inflammatory markers for predicting and diagnosing BPD. Compared with a single hematological parameter, SIRI and PNI complement the function of a single immune cell and can fully reflect the body’s inflammation and immune status.
SIRI is a comprehensive index based on peripheral blood NEUT, LYM, and MONO count. The increase of SIRI level indicates that the patient’s inflammatory response is strong, but the immune response is weak (16). Research by Cao et al. (14) and Cakir et al. (13) suggests that SIRI could serve as a valuable biomarker for predicting and diagnosing BPD, potentially aiding in the management of high-risk preterm infants, although data on its use in newborns remain limited. The study found an increase in SIRI levels among premature infants with BPD. Within 24 hours after birth, the SIRI showed predictive ability for msBPD, achieving an AUC of 0.599, with an optimal cut-off of 1.927, sensitivity at 57.1%, and specificity at 65.3%. At 36 weeks of PMA, the SIRI AUC for diagnosis msBPD in preterm infants was 0.602, with an optimal cut-off 5.175, sensitivity at 44.6%, and specificity at 80.0%. In summary, these results indicate that SIRI has the potential to predict the occurrence of msBPD within 24 hours after birth and assist in its diagnosis at 36 weeks of PMA. However, the predictive and diagnostic efficacy of SIRI is limited and warrants further investigation. In addition, the optimal cut-off value of SIRI is different from that in previous studies, which may be due to the different participants included in different studies.
PNI, an index combining serum ALB and LYM levels, indicates patients’ nutritional and immune status. In this study, PNI level was positively correlated with prognosis. The higher the PNI level, the better the prognosis of patients. Prior research indicates that PNI is linked to adult diseases, including malignant tumors, cardiovascular, autoimmune, and respiratory diseases (26-29). Limited information exists on the role of PNI in predicting and diagnosing diseases in newborns. Li et al. (15) investigated the value of PNI in predictive neonatal sepsis and found that PNI levels were reduced in neonates with sepsis and decreased significantly with the severity of sepsis. Huang et al. (24) conducted the only investigation into PNI in respiratory diseases affecting neonates; a significant association between low PNI levels (<46.875) and neonatal respiratory distress syndrome (NRDS) in preterm infants of different GAs was discovered. However, the relationship between PNI and the risk of BPD in preterm infants has not been reported. In this study, PNI was inversely proportional to the severity of BPD. The PNI demonstrated predictive capability for msBPD within 24 hours after birth, with an AUC of 0.588, an optimal cut-off value of 34.105, a sensitivity of 41.1%, and a specificity of 79.8%. At 36 weeks of PMA, the AUC of PNI for msBPD diagnosis in premature infants was 0.647, with an optimal cut-off value of 45.080, a sensitivity of 67.9%, and a specificity of 63.4%. To sum up, the PNI has the potential to predict the occurrence of msBPD within 24 hours after birth (<34.105) and assist in its diagnosis at 36 weeks of PMA (<45.080). Early diagnosis of msBPD has great significance for clinical practice. This study demonstrates that although the PNI holds some value in the early prediction and auxiliary diagnosis of msBPD, however, the sensitivity and specificity of PNI remain relatively low, potentially resulting in an increased rate of misdiagnosis. In this context, reducing the PNI threshold may elevate the false positive rate, thereby decreasing the likelihood of missed diagnoses to some extent. Nonetheless, this approach may also contribute to the overdiagnosis of msBPD. Consequently, in clinical practice, it is imperative for physicians to strike a balance between diagnostic sensitivity, specificity, and the overall well-being of premature infants.
Our study suggests that SIRI and PNI may be better indicators of BPD severity than simply counting NEUT, MONO, and LYM. The acquisition of SIRI/PNI is convenient and fast, and does not increase the cost of hospitalization and time, which improves the accuracy of clinicians in the early diagnosis of BPD. For premature infants with abnormal SIRI/PNI in the early postnatal period with high risk of msBPD, individualized and comprehensive management strategies should be implemented, such as ventilation therapy, infection prevention, and nutrition management, to reduce the incidence of msBPD and its associated severe sequelae (30). However, due to the limited research on SIRI and PNI in premature infants and the lack of reference thresholds, its practical application value needs to be proved by more studies.
There are some limitations to this study: (I) as a single-center study, the results of this study only reflect the local situation, and its practical application value needs to be validated through more studies; (II) this study only retrospectively analyzed preterm infants with GA <32 weeks. The sample size of the included cases is small, and there may be some bias. It is necessary to further expand the clinical sample size and multi-center prospective studies for verification.
ConclusionsOther Section
This study mainly evaluated systemic inflammatory indices (including SIRI and PNI) in the early postnatal period and 36 weeks of PMA, with the aim to identify potential biomarkers for predicting and diagnosing BPD. However, the predictive and diagnostic efficacy of SIRI and PNI is limited and warrants further investigation. It was initially discovered that PNI could serve as a predictive and diagnostic tool for evaluating the severity of BPD. In clinical practice, premature infants with low PNI need to be closely monitored, and countermeasures should be taken as early as possible to prevent the occurrence and progression of BPD.
AcknowledgmentsOther Section
None.
FootnoteOther Section
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-381/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-381/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-381/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-381/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Review Board (IRB) of The First Affiliated Hospital of Xinjiang Medical University granted ethical approval (No. K202212-12). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Cao Y, Jiang S, Sun J, et al. Assessment of Neonatal Intensive Care Unit Practices, Morbidity, and Mortality Among Very Preterm Infants in China. JAMA Netw Open 2021;4:e2118904. [Crossref] [PubMed]
- Bonadies L, Cavicchiolo ME, Priante E, et al. Prematurity and BPD: what general pediatricians should know. Eur J Pediatr 2023;182:1505-16. [Crossref] [PubMed]
- Cui X, Fu J. Reinitiating lung development: a novel approach in the management of bronchopulmonary dysplasia. Respir Res 2024;25:384. [Crossref] [PubMed]
- Branescu I, Shetty S, Richards J, et al. Pulmonary hypertension in preterm infants with moderate-to-severe bronchopulmonary dysplasia (BPD). Acta Paediatr 2023;112:1877-83. [Crossref] [PubMed]
- Li W, Wang Y, Song J, et al. Association between bronchopulmonary dysplasia and death or neurodevelopmental impairment at 3 years in preterm infants without severe brain injury. Front Neurol 2023;14:1292372. [Crossref] [PubMed]
- Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ 2021;375:n1974. [Crossref] [PubMed]
- Schmidt AR, Ramamoorthy C. Bronchopulmonary dysplasia. Paediatr Anaesth 2022;32:174-80. [Crossref] [PubMed]
- Mižíková I, Thébaud B. Perinatal origins of bronchopulmonary dysplasia-deciphering normal and impaired lung development cell by cell. Mol Cell Pediatr 2023;10:4. [Crossref] [PubMed]
- Heydarian M, Schulz C, Stoeger T, et al. Association of immune cell recruitment and BPD development. Mol Cell Pediatr 2022;9:16. [Crossref] [PubMed]
- Guo Y, Liu Y, Zhang R, et al. Analysis of variable metabolites in preterm infants with bronchopulmonary dysplasia: a systematic review and meta-analysis. Ital J Pediatr 2024;50:246. [Crossref] [PubMed]
- Schiller EA, Cohen K, Lin X, et al. Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates. Int J Mol Sci 2023;24:2622. [Crossref] [PubMed]
- Jiang J, Mao Y, Wu J, et al. Relationship between hematological parameters and bronchopulmonary dysplasia in premature infants. J Int Med Res 2023;51:3000605231187802. [Crossref] [PubMed]
- Cakir U, Tayman C, Tugcu AU, et al. Role of Systemic Inflammatory Indices in the Prediction of Moderate to Severe Bronchopulmonary Dysplasia in Preterm Infants. Arch Bronconeumol 2023;59:216-22. [Crossref] [PubMed]
- Cao L, Liu X, Sun T, et al. Predictive and Diagnostic Values of Systemic Inflammatory Indices in Bronchopulmonary Dysplasia. Children (Basel) 2023;11:24. [Crossref] [PubMed]
- Li T, Qi M, Dong G, et al. Clinical Value of Prognostic Nutritional Index in Prediction of the Presence and Severity of Neonatal Sepsis. J Inflamm Res 2021;14:7181-90. [Crossref] [PubMed]
- Islam MM, Satici MO, Eroglu SE. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: An extensive literature review. Turk J Emerg Med 2024;24:8-19. [Crossref] [PubMed]
- Jensen EA, Dysart K, Gantz MG, et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants. An Evidence-based Approach. Am J Respir Crit Care Med 2019;200:751-9. [Crossref] [PubMed]
- Wang J, Yang G, Cai Z, et al. Point prevalence, characteristics and treatment variations for preterm infants with bronchopulmonary dysplasia in China: a 'snapshot' study. BMJ Paediatr Open 2024;8:e002878. [Crossref] [PubMed]
- Siffel C, Hirst AK, Sarda SP, et al. The clinical burden of extremely preterm birth in a large medical records database in the United States: Mortality and survival associated with selected complications. Early Hum Dev 2022;171:105613. [Crossref] [PubMed]
- Glaser K, Jensen EA, Wright CJ. Prevention of Inflammatory Disorders in the Preterm Neonate: An Update with a Special Focus on Bronchopulmonary Dysplasia. Neonatology 2024;121:636-45. [Crossref] [PubMed]
- Karenberg K, Hudalla H, Frommhold D. Leukocyte recruitment in preterm and term infants. Mol Cell Pediatr 2016;3:35. [Crossref] [PubMed]
- Suzuki-Inoue K, Tsukiji N. Platelet CLEC-2 and lung development. Res Pract Thromb Haemost 2020;4:481-90. [Crossref] [PubMed]
- Yan L, Ren Z, Wang J, et al. The Correlation Between Bronchopulmonary Dysplasia and Platelet Metabolism in Preterm Infants. Front Pediatr 2021;9:670469. [Crossref] [PubMed]
- Huang L, Chen X, Zhang Y. Low Prognostic Nutritional Index (PNI) Level is Associated with an Increased Risk of Neonatal Respiratory Distress Syndrome in Preterm Infants with Different Gestational Ages: A Retrospective Study. Int J Gen Med 2024;17:5219-31. [Crossref] [PubMed]
- Kim S, McClave SA, Martindale RG, et al. Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship? Am Surg 2017;83:1220-7. [Crossref] [PubMed]
- Yang J, Li H, Li L, et al. Prognostic Role of Pretreatment Prognostic Nutritional Index in Advanced Lung Cancer Patients Receiving First-Line Immunotherapy: A Meta-Analysis. Cureus 2024;16:e52720. [Crossref] [PubMed]
- Zhang X, Su Y. Low Prognostic Nutritional Index Predicts Adverse Outcomes in Patients With Heart Failure: A Systematic Review and Meta-analysis. Angiology 2024;75:305-13. [Crossref] [PubMed]
- Pan L, Peng Y, Jiang L. Association between prognostic nutritional index and stroke: A nationally representative cross-sectional study from NHANES. J Stroke Cerebrovasc Dis 2025;34:108165. [Crossref] [PubMed]
- Stephenson SS, Kravchenko G, Korycka-Błoch R, et al. How Immunonutritional Markers Are Associated with Age, Sex, Body Mass Index and the Most Common Chronic Diseases in the Hospitalized Geriatric Population-A Cross Sectional Study. Nutrients 2024;16:2464. [Crossref] [PubMed]
- Villosis MFB, Barseghyan K, Ambat MT, et al. Rates of Bronchopulmonary Dysplasia Following Implementation of a Novel Prevention Bundle. JAMA Netw Open 2021;4:e2114140. [Crossref] [PubMed]
(English Language Editor: J. Jones)


