
Ultrasonographic diagnosis of secondary cardiac position alterations led by fetal thoraco-abdominal anomalies: a report of three cases
Highlight box
Key findings
• There is difficulty in distinguishing between congenital diaphragmatic hernia (CDH) and congenital diaphragmatic eventration (CDE) during the fetal period, both of which can lead to significant changes in heart position and lung dysplasia.
What is known and what is new?
• Prenatal ultrasound is important for diagnosing CDH and CDE.
• This study emphasizes the necessity for detailed ultrasound examination to identify the presence of liver in the thoracic cavity and the structural integrity of the diaphragm.
What is the implication, and what should change now?
• Accurate diagnosis is crucial for clinical management and prognosis, highlighting the role of ultrasound in guiding timely intervention and genetic testing.
• The implementation of prenatal care protocols should be improved to potentially reduce morbidity and mortality associated with CDH and severe CDE.
IntroductionOther Section
Congenital diaphragmatic hernia (CDH) and congenital diaphragmatic eventration (CDE) are rare congenital anomalies. They occur in approximately one in 2,000–3,000 and 2,500–5,000 children respectively (1,2). CDH is a defect in the diaphragm that allows movement of the contents of the abdominal cavity into the thoracic cavity. CDE is an abnormal development of muscle tissue in the embryonic transverse septum between 8 and 10 weeks of gestation. This condition can result in a fibrous membrane bulge caused by varying degrees of defects in muscle fibers or collagen fibers and the abnormal protrusion of abdominal organs into the thoracic cavity, which affects lung development (3). In contrast to CDH, there is no physical defect in the diaphragm, and the abdominal-thoracic cavities are separated in cases of CDE. CDE is always diagnosed by fluoroscopic examination. Although fluoroscopy is considered the gold standard for assessing diaphragmatic movement, it requires the patient to be transported to the radiology department and exposed to hypothermia and ionising radiation. Ultrasound can be performed safely at the bedside (4). It is difficult to distinguish CDE from right CDH because both can cause changes in position of the heart and lead to right lung hypoplasia; however, the management and prognosis of these two conditions differ greatly after birth (5,6). Here, we report three patients with abnormal positions of the heart. Among them was one case of right CDH (the right lobe of the liver) and one case of right CDE. The conditions in these two fetuses had typical sonographic manifestations and were definitively diagnosed by prenatal ultrasound, which provided an important basis for prenatal consultations and clinical management. In the remaining patient, the secondary dextroversion of the heart accompanied by persistent left superior vena cava (PLSVC) due to right lung agenesis was misdiagnosed as primary dextroversion of the heart on prenatal ultrasound. We present this case report in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-198/rc).
Case presentationOther Section
The first case involved a 26-year-old pregnant woman, G2P1. Her first child is alive. The patient’s spouse had drinking and smoking habits. Family history of congenital heart disease was denied. Prenatal screening showed low-risk of non-invasive DNA and Down’s syndrome. The patient received fetal echocardiography at our prenatal diagnosis center at her 24 weeks of gestation. The 4-chamber view on ultrasound (Figure 1A) revealed a large abnormal solid intermediate echo in the right thoracic cavity (herniation of the right lobe of the liver into the right thoracic cavity). The echo was thicker than that shown on the lung tissue photo points and caused an extreme shift of the heart toward the left thoracic cavity, with increased axial angles and compression of the right and left atria. The right atrium (Figure 1B, indicated by the line) was connected to the inferior vena cava and extended above the level of the diaphragm, and color Doppler flow confirmed the typical triphasic waveform of the inferior vena cava, namely, the atrial contraction wave, the ventricular contraction wave, and the ventricular diastolic wave. The hepatic veins (HV) drained into the inferior vena cava (Figure 1C). The amniotic fluid volume was normal. Thus, a diagnosis of right CDH (the right lobe of the liver) was made according to prenatal ultrasound. Patient terminated pregnancy at 24 weeks of gestation and was induced with a female stillbirth. The diagnosis was later confirmed by fetal autopsy (Figure 1D).

The second case involved a 31-year-old pregnant woman, G2P0, first miscarriage. The patient’s spouse had no alcohol or smoking habits, family history of congenital heart disease was denied, and prenatal screening of non-invasive DNA and Down’s syndrome showed low-risk. The patient at 25 weeks of gestation was transferred to our center to receive fetal echocardiography. The 4-chamber view on ultrasound revealed an abnormal solid intermediate echo in the right thoracic cavity (liver). The diaphragm had protruded into the right thoracic cavity, with the heart shifted leftwards (Figure 2A). The coronal view of the bilateral thoracoabdominal diaphragm revealed that the right and left diaphragms were not on the same level, with the right diaphragm being significantly elevated (Figure 2B). The right lung tissue was compressed, and the slender boundary between the supra- and subdiaphragm on the right side was also visible. The fetal lung was not expanded during the fetal period and no pulmonary circulation was established, only receiving a small amount of blood supply from the pulmonary artery. After the pregnancy was terminated at 25 weeks of gestation, an autopsy of the fetus showed that the right diaphragm had protruded into the right thoracic cavity. The diaphragm was thin, and the right lung was hypoplastic due to the compression (Figure 2C). When the right diaphragm was lifted, it was observed that part of the liver had protruded into the right thoracic cavity (Figure 2D), forming a CDH, thus the condition presented with both CDE and CDH. The fetal autopsy findings confirmed our prenatal ultrasound diagnosis.

The third case involved a 23-year-old pregnant woman, G1P0. The patient’s spouse had drinking and smoking habits. Family history of congenital heart disease was denied. Prenatal screening of non-invasive DNA and Down’s syndrome showed low-risk. The patient was transferred to our center to receive fetal echocardiography at her 28 weeks of gestation. The transverse abdominal view showed that the stomach vesicle was located on the left side of the abdominal cavity (Figure 3A). The 4-chamber cardiac view showed that the fetal cardiac axis pointed to the lower right side of the thoracic cavity (Figure 3B). Only the left pulmonary artery was seen on the right and left pulmonary artery views (Figure 3C), and the autopsy results confirmed the absence of the right lung and the right pulmonary artery (Figure 3D). The right hemithorax was empty with no right lung tissue present, and the left lung tissue was well-developed. The heart was rotated and displaced towards the empty right hemithorax. Additionally, there was a PLSVC (Figure 3D). This third case demonstrated dextrocardia, also known as isolated dextrocardia, which can be secondary or primary; in this case, it was secondary. The echocardiographic findings showed that the heart was located on the right side of the thorax, but the visceral positions were normal, i.e., the gastric bubble was on the left side of the abdomen, and the liver was on the right side. In contrast, true dextrocardia, or mirror-image dextrocardia, involved not only the heart being located in the right thoracic cavity but also a complete reversal of the thoracic (lungs and bronchi) and abdominal organ positions. Patient terminated pregnancy at 28 weeks of gestation and was induced with a female stillbirth. The autopsy showed right lung agenesis, which led to secondary dextroversion of the heart accompanied by PLSVC.
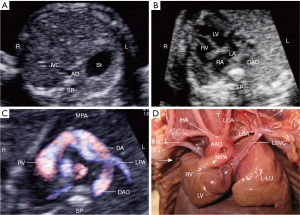
Among these three fetuses, one fetus had right CDH accompanied by a ventricular septal defect (VSD), one had CDE but no cardiac abnormalities, and one had right lung agenesis and PLSVC. In all of the three fetuses, other systemic structural examinations showed normal results, and noninvasive DNA testing indicated a low risk for common chromosomal abnormalities.
In all the three cases where legal guardianship was required, all three pregnant women and their legal guardians chose to terminate the pregnancies and signed informed consent, as well as the consent for autopsy. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
DiscussionOther Section
The normal position of the stomach and heart, as determined by two-dimensional ultrasonography, is on the left side of the fetus. The cardiac axis, a crucial marker for evaluating intrathoracic heart placement defined by the line from the cardiac base to the apex, normally inclines to the left, with the majority of the heart and its apex situated in the left hemithorax. Abnormalities in cardiac position frequently result from conditions such as pulmonary agenesis or hypoplasia, diaphragmatic hernias, among others. A normal cardiac axis angle measures approximately 45°±20°. These deviations in cardiac positioning have significant implications for diagnostic differentiation and clinical management.
Secondary abnormal cardiac position in fetuses often occurs due to mediastinal displacement secondary to diaphragmatic hernia, pulmonary cystic adenoma, and sequestration of the lung. Here, it is essential to differentiate between CDH and pulmonary cystic adenomatoid malformations, as accurate diagnosis is crucial for clinical management and prognosis.
Fetal CDH is a congenital condition characterized by the underdevelopment of the diaphragm, leading to the herniation of abdominal contents into the thoracic cavity and subsequent pulmonary hypoplasia. Pulmonary cystic adenomatoid malformations are categorized into three types: Type I with large cysts approximately 2 cm in diameter; Type II with cysts smaller than 2 cm; and Type III with microcystic patterns. The key to differentiating CDH from adenomatoid malformations lies in the presence of multiple cystic areas within the lung in the latter condition. In cases where the hernia content of CDH is the stomach bubble, and a large cystic dark area is seen in the left thoracic cavity with no stomach bubble structure observed in the left abdominal cavity below the diaphragm, it is easy to rule out pulmonary adenomatoid malformations. When the hernia content is at the liver, the sonographic appearance shows a slightly higher echogenicity in the right thoracic cavity compared to that of the lungs. Both CDH and pulmonary cystic adenomatoid malformations can cause abnormal cardiac positions to varying degrees. Scimitar syndrome refers to a condition where the right pulmonary veins or all pulmonary veins abnormally drain into the inferior vena cava in a scimitar-like pattern, coursing to the left. In these cases, the entry of the right pulmonary veins into the left atrium is absent from the posterior wall of the left atrium. When all pulmonary veins anomalously drain into the inferior vena cava, both the left and right pulmonary veins do not enter the left atrium, leading to a reduction in blood flow to the left atrium, causing a decrease in its size. However, the cardiac position within the thoracic cavity remains normal. In contrast, conditions such as right diaphragmatic hernia and right diaphragmatic eventration cause extrathoracic organs to protrude into the right chest cavity, pushing the normally left-positioned heart further to the left, resulting in an extreme leftward deviation of the heart axis. Right lung agenesis leads to dextroposition of the heart due to the subsequent shift of the heart towards the vacant right hemithorax, with the heart axis directed to the right, while maintaining normal visceral positions.
Following the discussion on differential diagnoses, it is pertinent to address the classification of dextrocardia, particularly in the context of our third case which presented with this condition. Classification of dextrocardia includes mirror-image dextrocardia and dextrocardia with situs solitus. In mirror-image dextrocardia, there is a reversal of the thoracic and abdominal viscera, placing the stomach on the right and the liver on the left. Isolated dextrocardia, on the other hand, is characterized by the heart being positioned on the right side with the abdominal viscera in their normal left-sided positions. However, in this instance, it describes a secondary form of dextrocardia implying that the cardiac malposition is a result of another underlying cause or pathology.
Early diaphragmatic herniation is associated with the intrusion of a greater number of abdominal organs and a more severe impact on lung development, resulting in congenital cardiac hypoplasia and a poor prognosis. The prognosis can be even worse if the fetus has other malformations and chromosomal abnormalities. In most cases, a right diaphragmatic hernia that protrudes into the thoracic cavity is the right hepatic lobe. The echogenicity of the liver parenchyma is similar to that of lung tissue, and an incautious scan of the positions of the left and right diaphragms can easily result in a missed diagnosis. Bahauddin et al. and Shwaartz et al. also reported that CDE is a rare disease that can be misdiagnosed as CDH (7,8). Among our cases, ultrasound in one fetus with right diaphragmatic hernia showed that both the inferior vena cava and hepatic veins travelled above the diaphragm, demonstrating that the solid echo in the thoracic cavity was due to liver herniation. Notably, both right CDH and right CDE can lead to an altered heart position and right lung hypoplasia, which sometimes makes differential diagnosis on sonogram particularly difficult. One fetus was misdiagnosed by prenatal ultrasound with a primary dextroversion of the heart accompanied by anomalous origin of the right pulmonary artery; after the termination of pregnancy, autopsy confirmed the diagnosis of dextroversion of the heart secondary to congenital right lung agenesis. Thus, when the right lung is hypoplastic or absent, the right intrathoracic cavity is empty, which may manifest as a malrotation and shift of the heart on ultrasound (9). The pathogenesis of fetal lung deficiency is unclear, and some scholars speculate that it is caused by abnormalities in pulmonary vasculature. The pulmonary artery evolved from the sixth pair of aortic arches in the early embryo. If there is inconsistency in position and connection during development, it will cause pulmonary artery stenosis or absence, and further lead to pulmonary dysplasia or absence (10). Currently, ultrasound technology is the preferred diagnostic method for the aforementioned cases, as it can detect the pathological changes in the thoracic and abdominal organs that lead to alterations in the position of the heart. If financial situation permits, magnetic resonance (MR) examination can be used as an auxiliary diagnostic tool. Depending on the severity of the condition and respecting the choice of the pregnant woman and the family, those with milder conditions may opt for emergency surgery after delivery. If there are additional malformations in other parts and chromosomal abnormalities, the prognosis is even poorer. Therefore, a search for lesions in the heart, lungs, diaphragm, abdominal cavity, and other structures is required when an abnormality is detected.
ConclusionsOther Section
CDH and severe CDE can affect the respiratory, circulatory, and digestive systems, and thus prenatal ultrasound is important for the timely diagnosis and proper management of these two conditions. CDH causes considerable mortality and usually requires emergency surgery after delivery (1). The related mortality reported in the literature is between 50% and 60% (11). When prenatal ultrasound reveals an abnormal fetal heart position, further detailed examinations of the heart as well as other thoracic and abdominal organs should be performed. Genetic testing technology have made it possible to obtain an earlier diagnosis in many cases with CDH (12). The continuity, positions, and local bulging (if any) of the bilateral diaphragm should be observed on the coronal view of the thoracoabdominal diaphragm while the structural relationships among the abdominal organs should be observed on the sagittal view so as to rule out any cause of the primary abnormal position of the heart. Primary alerts cannot be neglected and currently reported risk factors including prematurity, weight <2 kg, cyanosis at presentation and antenatal detected CDH or eventration (13) should be noted.
AcknowledgmentsOther Section
None.
FootnoteOther Section
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-198/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-198/prf
Funding: The study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-198/coif). L.Y. has received funding from the Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2015211C205). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Clifton MS, Wulkan ML. Congenital Diaphragmatic Hernia and Diaphragmatic Eventration. Clin Perinatol 2017;44:773-9. [Crossref] [PubMed]
- Gerall CD, Stewart LA, Price J, et al. Long-term outcomes of congenital diaphragmatic hernia: A single institution experience. J Pediatr Surg 2022;57:563-9. [Crossref] [PubMed]
- Zani A, Chung WK, Deprest J, et al. Congenital diaphragmatic hernia. Nat Rev Dis Primers 2022;8:37. [Crossref] [PubMed]
- Hoshino Y, Arai J. Diaphragm ultrasound examination for congenital diaphragmatic eventration in two premature neonates. BMJ Case Rep 2020;13:e232813. [Crossref] [PubMed]
- Sathasivam R, Bussa G, Viswanath Y, et al. ‘Mesh hiatal hernioplasty’ versus ‘suture cruroplasty’ in laparoscopic para-oesophageal hernia surgery; a systematic review and meta-analysis. Asian J Surg 2019;42:53-60. [Crossref] [PubMed]
- Evman S, Tezel C, Vayvada M, et al. Comparison of Mid-Term Clinical Outcomes of Different Surgical Approaches in Symptomatic Diaphragmatic Eventration. Ann Thorac Cardiovasc Surg 2016;22:224-9. [Crossref] [PubMed]
- Sallout B, Alshebli D, Sallout L, et al. Fetal Diaphragmatic Eventration: A Case Report. J Obstet Gynaecol Can 2021;43:993-7. [Crossref] [PubMed]
- Shwaartz C, Duggan E, Lee DS, et al. Diaphragmatic eventration presenting as a recurrent diaphragmatic hernia. Ann R Coll Surg Engl 2017;99:e196-9. [Crossref] [PubMed]
- Lavender I. Twining’s textbook of fetal abnormalities – 3rd edition. Sonography 2016;3:37.
- Cotten CM. Pulmonary hypoplasia. Semin Fetal Neonatal Med 2017;22:250-5. [Crossref] [PubMed]
- Brownlee EM, Howatson AG, Davis CF, et al. The hidden mortality of congenital diaphragmatic hernia: a 20-year review. J Pediatr Surg 2009;44:317-20. [Crossref] [PubMed]
- Scott DA, Gofin Y, Berry AM, et al. Underlying genetic etiologies of congenital diaphragmatic hernia. Prenat Diagn 2022;42:373-86. [Crossref] [PubMed]
- Vasudev RB, Kumar N, Gadgade BD, et al. Factors contributing to mortality in neonates with congenital diaphragmatic hernia and eventration. Afr J Paediatr Surg 2023;20:85-8. [Crossref] [PubMed]
(English Language Editor: J. Gray)



