
Age- and sex-specific reference intervals for sex hormones in children in Wuhan: a cross-sectional study of 2,477 healthy children and adolescents
Highlight box
Key findings
• This study established age- and sex-specific pediatric reference intervals (RIs) for estradiol (E2), follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), progesterone (PROG), and testosterone (TESTO) from birth to 19 years in children and adolescents in Wuhan, China.
What is known and what is new?
• The age- and sex-dependent level of sex hormones, which changes throughout various stages from neonates to adults, is an essential indicator for assessing children’s growth and development and plays a significant role in clinical decision-making.
• This study established the age- and sex-specific RIs for sex hormones, including E2, FSH, LH, PRL, PROG, and TESTO, for a Chinese pediatric and adolescents population consisting of 2,477 healthy participants (from birth to 19 years old) on the Mindray CL-6000i automated chemiluminescence immunoassay analyzer.
What is the implication, and what should change now?
• This study established age- and sex-specific RIs for sex hormones on the Mindray CL-6000i platform, enabling effective laboratory use. These RIs can facilitate accurate and prompt result interpretation for clinicians.
• The impact of tanner staging and body mass index (BMI) on the RIs of sex hormones among children aged 9–15 years remains to be determined, which is an area we intend to address in future research.
• The diagnostic accuracy, sensitivity, and specificity of these RIs for endocrine disorders in clinical practice require further elucidation and enhancement.
Introduction
Reference intervals (RIs) are defined as the range of values from the 0.025 to 0.975 quantiles (encompassing 95% of normal results), including the limiting values themselves, observed in apparently healthy individuals (1). An accurate RI plays a crucial role in health assessments, disease diagnosis, prognosis judgments, and observations of curative effects in clinical patients (2). However, numerous existing RIs are based on outdated methods and consequently lags behind the current standards or prove to be inadequate, particularly in the context of pediatric references (3). Pediatric RIs differ from those of adults due to the significant differences in growth, development characteristics, and physiological functions between children and adults (4,5). This is especially apparent for parameters that are associated with children’s growth and differentiation, such as hormones in the hypothalamic-pituitary-gonadal (HPG) axis.
In children, the HPG axis hormones are critically involved in growth and sexual differentiation (3,6). Determinations of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), prolactin (PRL), progesterone (PROG), and testosterone (TESTO) levels are indispensable in functional evaluations of the HPG axis (7,8). FSH and LH regulate the development of reproductive organs and the secretion of sex hormones (3) while E2 promotes the development of female sexual characteristics (9). PRL stimulates breast development (10), PROG contributes to menstrual cycle control, and TESTO promotes the development of male sexual characteristics (11). These hormones do not operate independently but rather exist within a complex network of interactions and regulatory mechanisms. Together, they maintain the endocrine system’s stability and equilibrium in children, thereby ensuring normal growth and development (5,6). Prior to puberty, gonadal growth and development proceed slowly, with the HPG axis operating at a relatively basal level. However, once puberty begins, the frequency and peak of the pulsed secretion of gonadotropin-releasing hormone (GnRH) gradually increases and is accompanied by an increase in the pulsed secretion peaks of LH and FSH, leading to the development of sexual characteristics and organs (6). Clinically, disorders of sex development (DSD) encompass abnormalities in gonadal, secondary sexual characteristics, and sexual function that arise due to various reasons after birth. These abnormalities include precocious puberty, delayed puberty, and infantilism (12,13). In precocious puberty, individuals exhibit sex hormone levels in their blood that significantly exceed the normal range for their age and gender, often reaching or even surpassing adolescent or adult levels (12,13).
Sex hormones are essential indicators for assessing the growth and development status of children, playing a significant role in clinical decision-making (14). However, various factors such as age, Tanner stage, ethnicity, anthropometric characteristics, detection methodology, and other determinants can influence the RIs of these hormones (15). Therefore, using adult sex hormones standards or RIs from other countries for children may lead to misdiagnosis or missed diagnosis of childhood sexual developmental disorders, thereby compromising the accuracy of diagnosing and treating pediatric diseases in China. Unfortunately, few laboratories in China currently provide reliable RIs for children’s sex hormones (10,16-18). Without adequate RIs, there is an increased risk of missing important pathological changes and an increased risk of erroneously interpreting normal changes as pathological. Given that physiologic hormone levels are closely related to age and sex during childhood (4), it is imperative to employ accurate reference values based on sex and age for a precise understanding of test results. The dynamic physiology associated with childhood and adolescence often necessitates the establishment of multiple partitions specific to age and sex, significantly increasing the required sample size.
Establishing RIs of pediatric sex hormones is challenging due to difficulties in obtaining sufficient samples, especially for younger subgroups such as newborns (9). This study thus aimed to evaluate the distribution of sex hormones and to establish precise, age- and sex-specific RIs that are tailored to the unique characteristics of a Chinese pediatric population. Serum E2, FSH, LH, PRL, PROG, and TESTO levels in 2,477 healthy Chinese children and adolescents (from birth to 19 years) were directly tested on the Mindray CL-6000i automatic chemiluminescence immunoassay analyzer. Following guideline C28-A3 of the Clinical Laboratory Standards Institute (CLSI) (19), we analyzed the hormone distribution and established accurate serum sex hormone RIs for Chinese children. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-399/rc).
Methods
Ethical approval
This study was conducted according to the Declaration of Helsinki (as revised in 2013) for research on human participants, and was approved by the institutional ethics board of Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology (No. 2024R042-E01). All methods and experimental protocols in this study were conducted following approved protocols and the ethics board’s existing guidelines. Written informed consent was obtained from all participants (provided by at least one guardian).
Sample size calculation
This a posteriori, direct method of reference value analysis was carried out according to the CLSI C28-A3 guideline (19). This guideline recommends an ideal minimum sample size of 120 healthy reference individuals per partition for RI calculation (95% RI, two-tailed) to obtain a robust estimate of the 90% confidence interval using nonparametric methods. In addition, at least 120 cases per group were required for grouping. For newborns and infants, a minimum sample size of at least 40 cases was recommended. The sex ratio overall and in each group was designated as 1:1.
Study population
The inclusion criteria for the reference population were as follows (20): The study population were selected by questionnaire survey and physical examination, and all the following criteria should be met: (I) healthy newborns who was born without obvious congenital anomalies, genetic diseases or acute infection symptoms, and whose birth weight, length, and Apgar score were within the normal range; (II) physical examination and laboratory test results within the reference range for children and adolescents; (III) no endocrine-related diseases or other conditions or medications that would cause changes in the levels of HPG axis function-related indicators (E2, FSH, LH, PRL, PROG, and TESTO). Meanwhile, the exclusion criteria were as follows (12): (I) any infant born prematurely or suspected of genital crisis infants; (II) children with a history of chronic illness (endocrine, inflammatory, autoimmune, cancer, and kidney diseases); (III) acute illness within the previous week, or use of prescribed or nonprescribed medication within the previous 2 weeks. Study participants were subjected to anthropometric measurements and collection of 2 mL of venous blood. Between September 2022 and August 2023, a total of 2,715 children and adolescents were recruited from the Physical Examination Center of Wuhan Children’s Hospital. To ensure the healthy and optimal condition of the study participants, we excluded children with incomplete medical histories (n=18), relevant drug usage (n=79), or a history of chronic diseases (n=73) or those whose consent was not provided by guardians or who withdrew from the study (n=25). During data cleaning phase, contaminated/unqualified specimens (n=18) and specimens beyond the detection range (n=25) were also excluded. After exclusion, 2,477 eligible participants were finally included in this study (Figure 1).

Serological testing and quality control
Venous blood (2 mL) was drawn from each participant, with most samples collected between 8:00 AM and 10:00 AM. As in our previous study (21), a Cobas P612 pretreatment system (Roche Diagnostics, Rotkreuz, Switzerland) was used to separate serum within 4 hours. A CL-6000i automated chemiluminescence immunoassay analyzer (Mindray Biomedical Electronics Co., Ltd., Shenzhen, China) was used to detect serum E2, FSH, LH, PRL, PROG, and TESTO levels. All dedicated reagents were provided by Shenzhen Mindray Biomedical Electronics Co., Ltd. The analytical method was strictly controlled by routine maintenance, calibration, and quality control according to the manufacturer’s instructions and clinical laboratory standards and routine operating protocols, and samples were analyzed only when all analytical parameters were acceptable. The linear range of the E2, FSH, LH, PRL, PROG, and TESTO kit were 25–4,800 pg/mL, 0.2–200 mIU/mL, 0.2–250 mIU/mL, 0.47–200 ng/mL, 0.1–40 ng/mL, 0.1–16 ng/mL.
Statistical analysis and determination of RI
Statistical analysis was performed using R version .3.6.3 and R-studio version 1.4.1106 (The R Foundation for Statistical Computing, Vienna, Austria). Following the recommendations of the CLSI EP9-A3 guideline (2), a descriptive analysis and an RI analysis were developed. Frequencies and percentages are used to describe the categorical variables. The Shapiro-Wilk test and skewness/kurtosis tests were used to test whether continuous data conformed to a normal distribution. Outliers were eliminated by combining the generalized extreme studentized deviation (ESD) method and box diagram (outlier ratio ≤5%) and subsequently eliminated. Age partitioning was based on visual evaluation, and subgroups were compared with Kruskal-Wallis analysis of variance and followed by a post hoc test. The Goodman-Kruskal gamma test was used for the distribution test in the age group × sex crosstabs. The “ggplott2” package in R was used to represent the probability density curves of sex hormones in the male, female, and total populations. A scatter plot with linear fitting for the distribution of sex hormones levels at different ages was constructed using GraphPad Prism 8 (GraphPad Software, CA, La Jolla, USA). The age division of the reference values of the detection indicators was performed using a piecewise regression analysis. All sex hormones indicators are presented as the 2.5th percentile (P2.5), median (P50), and 97.5th percentile (P97.5). All P values were two-sided, with P<0.05 indicating statistical significance.
Results
Basic characteristics of the study population
A total of 2,477 healthy individuals were included in this study and were divided into the following subgroups according to age: 1 day–1 month, >1–12 months, >1–3 years, >3–6 years, >6–9 years, >9–12 years, >12–15 years, >15–18 years, and >18–19 years. Based on the generalized ESD method and box diagram, 65 E2 values, 46 FSH values, 95 LH values, 60 PRL values, 119 PROG values, and 95 TESTO values were eliminated as outliers (Figure 1). The number and proportion of abnormal values for E2, FSH, LH, PRL, PROG, and TESTO in the different age groups are shown in Table S1. The frequency distribution of each index, after the exclusion of outliers, is shown in Table 1 for the different age groups. The age distribution was approximately equal between the male and female groups, except for 19-year-old participants.
Table 1
| Age group | Gender | E2 (pg/mL) | FSH (mIU/mL) | LH (mIU/mL) | PRL (ng/mL) | PROG (ng/mL) | TESTO (ng/mL) |
|---|---|---|---|---|---|---|---|
| 1 d–1 m | Female (%) | 70 (41.92) | 64 (39.51) | 71 (42.51) | 72 (42.6) | 70 (43.21) | 70 (42.94) |
| Male (%) | 97 (58.08) | 98 (60.49) | 96 (57.49) | 97 (57.4) | 92 (56.79) | 93 (57.06) | |
| >1–12 m | Female (%) | 79 (35.59) | 75 (34.88) | 80 (36.04) | 78 (36.45) | 76 (35.85) | 80 (37.38) |
| Male (%) | 143 (64.41) | 140 (65.12) | 142 (63.96) | 136 (63.55) | 136 (64.15) | 134 (62.62) | |
| >1–3 y | Female (%) | 70 (50.0) | 66 (48.53) | 67 (50.38) | 70 (50.0) | 68 (50.75) | 68 (50.75) |
| Male (%) | 70 (50.0) | 70 (51.47) | 66 (49.62) | 70 (50.0) | 66 (49.25) | 66 (49.25) | |
| >3–6 y | Female (%) | 106 (50.96) | 104 (50) | 103 (51.5) | 103 (50.74) | 102 (49.76) | 104 (52.0) |
| Male (%) | 102 (49.04) | 104 (50) | 97 (48.5) | 100 (49.26) | 103 (50.24) | 96 (48.0) | |
| >6–9 y | Female (%) | 264 (45.36) | 261 (45.31) | 251 (44.82) | 250 (44.64) | 245 (43.75) | 253 (45.1) |
| Male (%) | 318 (54.64) | 315 (54.69) | 309 (55.18) | 310 (55.36) | 315 (56.25) | 308 (54.9) | |
| >9–12 y | Female (%) | 155 (38.75) | 172 (41.25) | 154 (38.6) | 166 (40.79) | 157 (39.15) | 167 (41.85) |
| Male (%) | 245 (61.25) | 245 (58.75) | 245 (61.4) | 241 (59.21) | 244 (60.85) | 232 (58.15) | |
| >12–15 y | Female (%) | 99 (45.41) | 105 (46.67) | 102 (45.74) | 108 (47.16) | 92 (44.23) | 101 (45.29) |
| Male (%) | 119 (54.59) | 120 (53.33) | 121 (54.26) | 121 (52.84) | 116 (55.77) | 122 (54.71) | |
| >15–18 y | Female (%) | 107 (48.42) | 117 (51.32) | 112 (49.78) | 116 (50.43) | 110 (50.0) | 111 (49.12) |
| Male (%) | 114 (51.58) | 111 (48.68) | 113 (50.22) | 114 (49.57) | 110 (50.0) | 115 (50.88) | |
| >18–19 y | Female (%) | 30 (11.81) | 41 (15.53) | 30 (11.86) | 44 (16.6) | 33 (12.89) | 37 (14.12) |
| Male (%) | 224 (88.19) | 223 (84.47) | 223 (88.14) | 221 (83.4) | 223 (87.11) | 225 (85.88) | |
| Total | 2,412 (100.0) | 2,431 (100.0) | 2,382 (100.0) | 2,417 (100.0) | 2,358 (100.0) | 2,382 (100.0) | |
| Female (%) | 980 (40.63) | 1,005 (41.34) | 970 (40.72) | 1,007 (41.66) | 953 (40.42) | 991 (41.6) | |
| Male (%) | 1,432 (59.37) | 1,426 (58.66) | 1,412 (59.28) | 1,410 (58.34) | 1,405 (59.58) | 1,391 (58.4) |
E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; PROG, progesterone; TESTO, testosterone; m, month; y, year.
Distribution characteristics of sex hormones
The distribution of serum E2, FSH, LH, PRL, PROG, and TESTO levels was analyzed on a linear scale in the total, male, and female populations (Figure 2). All six hormones exhibited a nonnormal distribution, and thus the data are presented in binary percentiles (P2.5–P97.5). The reference range limits for E2, FSH, LH, PRL, PROG, and TESTO of males differed significantly from those of females (Table 2). Specifically, the reference range for TESTO was broader and higher in males compared to females, whereas the PRL range for males was wider but with lower values compared to that of females (P2.5–P97.5: 4.19–161.26 vs. 4.46–155.28 ng/mL; P50: 10.91 vs. 11.65 ng/mL; P=0.008). For E2, FSH, LH, and PROG, the reference ranges in females were higher than those in males. The sex- and age-specific distribution of serum E2, FSH, LH, PRL, PROG, and TESTO levels also confirmed the difference in reference ranges between males and females (Figure 3). In addition, all six hormones demonstrated a complex pattern of change in analyte concentrations between different age groups with notable differences between sexes (Tables S2,S3). Moreover, significant variations were observed in all six hormones levels during the first month of life, necessitating subdivisions within the first year (Table 3).

Table 2
| Group | E2 (pg/mL) | FSH (mIU/mL) | LH (mIU/mL) | PRL (ng/mL) | PROG (ng/mL) | TESTO (ng/mL) |
|---|---|---|---|---|---|---|
| Total | 25.26 [8.28, 73.86] | 2.81 [0.52, 9.63] | 0.39 [0.05, 11.47] | 11.20 [4.22, 156.00] | 0.46 [0.14, 3.66] | 0.06 [0.00, 7.71] |
| Male | 24.68 [7.03, 52.57] | 2.52 [0.47, 8.44] | 0.63 [0.05, 9.03] | 10.91 [4.19, 161.26] | 0.45 [0.14, 2.96] | 0.08 [0.00, 8.38] |
| Female | 25.97 [9.56, 121.68] | 3.39 [0.69, 10.40] | 0.24 [0.05, 14.96] | 11.65 [4.46, 155.28] | 0.47 [0.14, 6.16] | 0.04 [0.00, 0.66] |
| P | <0.001 | <0.001 | <0.001 | 0.008 | 0.001 | <0.001 |
Data were analyzed with the Kruskal-Wallis test. The P50 [P2.5, P97.5] for each sex hormone is presented. E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; PROG, progesterone; TESTO, testosterone; P50, median; P2.5, 2.5th percentile; P97.5, 97.5th percentile.

Table 3
| Age group | E2 (pg/mL) | FSH (mIU/mL) | LH (mIU/mL) | PRL (ng/mL) | PROG (ng/mL) | TESTO (ng/mL) |
|---|---|---|---|---|---|---|
| 1 d–1 m | 31.16 [15.72, 61.95] | 3.86 [0.42, 19.69] | 2.18 [0.09, 11.72] | 126.7 [5.58, 323.95] | 2.01 [0.16, 5.93] | 0.5 [0.00, 1.77] |
| >1–12 m | 22.83 [10.79, 39.25] | 2.84 [0.43, 13.72] | 0.43 [0.04, 6.36] | 17.69 [6.16, 66.83] | 0.50 [0.13, 3.43] | 0.02 [0.00, 0.98] |
| >1–3 y | 21.61 [8.15, 41.74] | 1.84 [0.41, 7.37] | 0.14 [0.06, 0.53] | 13.17 [4.95, 38.03] | 0.19 [0.09, 0.50] | 0 [0.00, 0.05] |
| >3–6 y | 20.21 [5.77, 33.73] | 1.68 [0.43, 4.48] | 0.16 [0.08, 0.44] | 9.86 [4.41, 23.31] | 0.44 [0.28, 0.88] | 0.02 [0.00, 0.07] |
| >6–9 y | 22.38 [7.43, 38.95] | 1.81 [0.54, 5.38] | 0.16 [0.03, 0.68] | 8.94 [4.21, 27.03] | 0.36 [0.14, 1.40] | 0.02 [0.00, 0.11] |
| >9–12 y | 23.82 [6.99, 53.09] | 2.82 [0.52, 8.18] | 0.35 [0.03, 3.55] | 8.65 [3.82, 22.11] | 0.39 [0.14, 0.87] | 0.05 [0.00, 0.27] |
| >12–15 y | 31.93 [3.97, 154.11] | 4.98 [1.73, 10.29] | 4.66 [0.85, 17.47] | 11.25 [0.94, 34.66] | 0.52 [0.18, 12.06] | 0.37 [0.05, 6.03] |
| >15–18 y | 41.06 [14.72, 210.1] | 4.85 [1.55, 10.44] | 5.37 [0.75, 19.57] | 13.45 [4.67, 37.93] | 0.62 [0.29, 13.11] | 0.84 [0.09, 8.74] |
| >18-19 y | 36.02 [18.69, 70.60] | 4.01 [1.58, 9.39] | 4.68 [2.09, 11.41] | 13.45 [6.68, 27.58] | 0.67 [0.33, 1.40] | 5.38 [0.23, 9.54] |
The P50 [P2.5, P97.5] for each sex hormone is presented. E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; PROG, progesterone; TESTO, testosterone; P50, median; P2.5, 2.5th percentile; P97.5, 97.5th percentile; m, month; y, year.
Pediatric RIs for sex hormones
Differences in serum E2, FSH, LH, PRL, PROG, and TESTO levels were observed across different age groups according to the Kruskal-Wallis analysis and Nemenyi test. Since the basal hormone values in prepubertal and adolescent children often overlap, groups with similar results were combined based on the test results to facilitate clinical application. The RIs for sex hormones in specific age and sex groups were calculated based on the results described above (Figure 4). The P50 and 95% distributions (P2.5–P97.5) of serum E2, FSH, LH, PRL, PROG, and TESTO in pediatric participants are presented in Table 4.
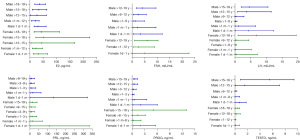
Table 4
| Variable | Male | Female | |||
|---|---|---|---|---|---|
| Age group | RI, P50 [P2.5, P97.5] | Age group | RI, P50 [P2.5, P97.5] | ||
| E2 (pg/mL) | 1 d–1 m | 31.08 [16.78, 61.53] | 1 d–1 m | 31.72 [13.51, 67.44] | |
| >1 m–12 y | 21.92 [6.92, 35.43] | >1 m–12 y | 23.14 [10.00, 48.01] | ||
| >12–15 y | 27.52 [2.77, 62.82] | >12–15 y | 45.56 [4.73, 168.04] | ||
| >15–18 y | 34.61 [10.05, 64.22] | >15–18 y | 52.66 [23.06, 224.44] | ||
| >18–19 y | 35.86 [19.31, 59.45] | >18–19 y | 43.13 [16.53, 111.50a] | ||
| FSH (mIU/mL) | 1 d–1 m | 3.43 [0.50, 12.02] | 1 d–1 y | 5.02 [0.04, 22.73] | |
| >1–12 m | 2.12 [0.39, 9.53] | >1–12 y | 2.69 [0.62, 9.00] | ||
| >1–9 y | 1.27 [0.42, 4.98] | >12–19 y | 6.01 [1.00, 10.38] | ||
| >9–12 y | 2.45 [0.50, 5.73] | ||||
| >12–19 y | 4.09 [1.68, 9.53] | ||||
| LH (mIU/mL) | 1 d–1 m | 4.40 [0.09, 12.27] | 1 d–1 m | 0.53 [0.09, 7.14] | |
| >1–12 m | 1.19 [0.05, 6.60] | >1–12 m | 0.18 [0.03, 5.21] | ||
| >1–9 y | 0.18 [0.04, 0.67] | >1–9 y | 0.14 [0.03, 0.51] | ||
| >9–12 y | 0.34 [0.03, 2.89] | >9–12 y | 0.35 [0.07, 4.44] | ||
| >12–15 y | 3.71 [0.83, 11.55] | >12–19 y | 6.79 [1.00, 19.49] | ||
| >15–19 y | 4.55 [2.00, 10.52] | ||||
| PRL (ng/mL) | 1 d–1 m | 132.64 [5.12, 322.45] | 1 d–1 m | 109.46 [5.91, 333.29] | |
| >1–12 m | 17.72 [6.12, 63.50] | >1–12 m | 17.56 [6.09, 70.31] | ||
| >1–3 y | 12.37 [4.77, 34.24] | >1–3 y | 13.45 [5.05, 40.20] | ||
| >3–9 y | 9.12 [4.16, 25.89] | >3–9 y | 9.18 [4.50, 26.51] | ||
| >9–19 y | 10.70 [3.49, 27.37] | >9–15 y | 10.51 [1.00, 30.77] | ||
| >15–19 y | 16.19 [6.29, 41.04] | ||||
| PROG (ng/mL) | 1 d–1 m | 2.05 [0.16, 6.04] | 1 d–1 m | 1.91 [0.21, 5.62] | |
| >1–12 m | 0.52 [0.12, 3.50] | >1 m–6 y | 0.38 [0.11, 2.11] | ||
| >1–3 y | 0.17 [0.08, 0.46] | >6–15 y | 0.44 [0.19, 3.43] | ||
| >3–12 y | 0.36 [0.14, 0.96] | >15–19 y | 0.73 [0.35, 14.24] | ||
| >12–15 y | 0.48 [0.19, 1.34] | ||||
| >15–19 y | 0.61 [0.31, 1.30] | ||||
| TESTO (ng/mL) | 1 d–1 m | 0.74 [0, 1.80] | 1 d–12 m | 0.10 [0.00, 0.74] | |
| >1 m–9 y | 0.02 [0.00, 0.75] | >1–12 y | 0.02 [0.00, 0.15] | ||
| >9–12 y | 0.04 [0.00, 0.30] | >12–18 y | 0.26 [0.04, 0.85] | ||
| >12–15 y | 1.55 [0.08, 7.11] | >18–19 y | 0.36 [0.12, 0.76] | ||
| >15–19 y | 5.53 [0.87, 9.47] | ||||
a, 95th percentile presented and 97.5th not available. RI, reference interval; E2, estradiol; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PRL, prolactin; PROG, progesterone; TESTO, testosterone; P50, median; P2.5, 2.5th percentile; P97.5, 97.5th percentile; m, month; y, year.
Immediately after birth, E2 serum concentration appeared high, but decreased rapidly within the first 30 days of life, showing no significant sex differences during this phase (P50: 31.08 vs. 31.72 pg/mL). Thereafter, E2 concentrations remained unaltered, with no observable difference between the sexes until the onset of puberty. During sexual maturation, a slight increase in E2 was observed in males, whereas the medians of E2 increased more significantly in females. During the first year of life, both LH and FSH levels were increased, with LH showing a more pronounced rise in males and FSH exhibiting a more significant elevation in females. Beginning with the age group of 12 years (i.e., with the onset of puberty), medians for both LH and FSH concentrations started to increase significantly, and the RIs for females were higher than those for males.
PRL exhibited higher concentrations in both sexes during the neonatal period, which subsequently began to decline after 30 days. However, due to the increase in PRL levels observed among females after the age of 12 years old, an additional RI group was established specifically for them, which was not established for males. During the neonatal period, both PROG and TESTO concentrations increased, with variations in the magnitude of this rise across sexes. Notably, the levels of PROG and TESTO were significantly higher in males compared to females during the early infancy period (1 month). PROG levels in females shows great fluctuation during the prepubertal and adolescent years (ages >1 month–19 years), leading to the consolidation of four RIs. Additionally, there was a notable increase in TESTO levels among boys over 12 years old.
Discussion
The level of sex hormones varies with age and gender, reflecting different developmental stages from neonates to adults (5,22). These hormones’ concentrations can indicate the developmental status of organs and tissues (12). In clinical practice, abnormal sex hormones levels in children and adolescents can be indicative of ambiguous genitalia in infants, hypogonadism, precocious puberty, oligomenorrhea, hirsutism in females, feminization in males, and, more rarely, neoplasms that produce estrogens or androgens (6,13). Given the crucial role of these hormones in sexual development, the established RIs for children should accurately reflect the dynamic physiological changes and patterns of each stage of child development. In laboratory practice, while manufacturer-provided RIs are commonly used, it is generally recommended that each laboratory establish its own RIs and update them whenever test methods or analyzers change or are upgraded (23). The considerable interstudy heterogeneity of sex hormone RIs in the pediatric population is the reason the derivation of these ranges are difficult and complex in children, so there is almost always a lack of pediatric references (24). Our study established age- and sex-specific RIs for sex hormones, including E2, FSH, LH, PRL, PROG, and TESTO for a Chinese pediatric and adolescents population consisting of 2,477 healthy participants (from birth to 19 years old) on the Mindray CL-6000i automated chemiluminescence immunoassay analyzer. The number of participants included in our investigation has been much greater than in previously published studies on sex hormone reference values.
In this study, we employed a chemiluminescence method to analyze trends in the levels of six sex hormones across various age groups in healthy children and adolescents. The results indicated statistically significant differences in sex hormone levels among these age groups. Specifically, from the neonatal period to infancy, sex hormone levels declined to varying degrees, followed by a sustained period of low concentrations. Notably, upon entering puberty, the levels of FSH, LH, E2, and TESTO increased rapidly, except for PROG and PRL, which did not exhibit an increase during this period. Comparison across age groups revealed that the levels of these four sex hormones were significantly higher in both the neonatal and pubertal groups compared to the other age groups. The RI of sex hormones that we have established for Chinese children and adolescents aligns with the dynamic shifts in sex hormones during adolescence. During the growth process of the human fetus from various stages of development to adolescence and full sexual maturity, the regulation and initiation of the HPG axis play an important role, and hormones also accordingly undergo a series of changes (6). The HPG axis is primarily modulated by two distinct mechanisms. First, there is a sex hormone-dependent negative feedback regulatory mechanism. Usually, once a certain threshold level of sex hormone is reached, it can suppress the secretion of GnRH and gonadotropin, which is particularly prominent at 2–3 years of age (5,25). Second, the central nervous system (CNS) exerts an inhibitory influence on the HPG axis. For example, adrenergic agonists can stimulate GnRH release, while endogenous enkephalin can inhibit GnRH release. This CNS-mediated regulatory mechanism mainly comes into effect in the age range of 3–10 years (9).
This study revealed that sex hormone levels in infants from birth to 1 year is high. In the fetal period, due to the massive secretion of sex hormones from the placenta and the inhibitory function of the fetal CNS, the HPG axis is functional by 10 weeks’ gestation and remains active until early infancy when it enters into a relatively quiescent phase (5,9). However, the sex hormone from the placenta is interrupted, and the negative feedback effect is weakened following birth, so the secretion of gonadotropin and sex hormone increases again and can last for approximately 6 months. A transient postnatal activation of the HPG axis, also termed minipuberty, occurs in healthy infants (6). The RI we established is consistent with the above pattern, and the results show that the increase in gonadotropins reaches its peak at 1 week to 3 months of age in infants, but appears to show a marked sexual dimorphism with the preponderance of LH in males and of FSH in females. However, The FSH values in the neonatal serum observed were similar to those reported in some earlier studies but higher than those found in others (3,4). Before puberty, children have already established a feedback connection between the hypothalamus, pituitary, and gonads, and the interaction is highly sensitive. The secretion of a small amount of hormone by immature gonads is sufficient to effectively inhibit the secretion of GnRH from the hypothalamus, LH from the adenohypophysis, and FSH. Therefore, the level of sex hormones in children before puberty remains very low, which suggests that additional inhibitory mechanisms in the CNS and hypothalamus prevent the induction of gonadotropin secretion from the pituitary gland.
Despite their relatively low levels during childhood, circulating estrogens play a pivotal role in the development and maintenance of the female phenotype, and they also significantly contribute to longitudinal growth, nervous system maturation, bone metabolism, and endothelial responsiveness, impacting both males and females alike (22,26). For prepubescent children, serum values of LH and FSH are essential biochemical markers used for the evaluation of suspicious disorders of sexual development. FSH promotes follicular development, and spermatogenesis, accelerates growth, and stimulates the development of secondary sexual characteristics. Notably, during the early stages of puberty, FSH increases earlier than LH, resulting in an increased FSH:LH ratio in prepuberty, which subsequently decreases in late puberty. In females, FSH increases after birth, stabilizes until the age of 1 year, and then declines significantly. The onset of sexual maturation in females is marked by an increase in gonadal activity, accompanied by elevated LH and FSH levels (7,27). The conclusion of this study agrees with it. Pediatric RIs for LH, FSH were determined in the study of Ivana Zec and co-workers; prepubertal children were separated by gender in the three age bands, from neonates to 4 years, 4 to 8 years and 8 to 11 years of age (9). In contrast, we have a more comprehensive age group and finer age segmentation, which is of great significance for the diagnosis and treatment evaluation of endocrine diseases such as precocious puberty in children. Unlike these two hormones, E2 exhibited minimal sexual differences during infancy. A slight increase was observed in both sexes during the first year of life, which was followed by a plateau that persisted until puberty (around age 9 for females and age 11 for males), even before any physical signs of puberty became apparent, with females showing significantly higher E2 levels compared to males. This is consistent with studies of Di Meo et al. and Rosner et al. (28,29). We hypothesize that the higher, albeit still low, E2 concentrations in prepubertal females compared to those of males are likely to serve a specific biological function and might contribute to the earlier onset of sexual maturation in females. Concentrations in children reached a peak concentration at 12–18 years of age. During sexual maturation, a slight increase in E2 was observed in males, while the medians of E2 increased more strongly in females. Similar to the previous Korean study, E2 levels were found to be increased dramatically in girls aged 12 years (30). Five age partitions were required for E2 RI in both sexes. The measurement of E2 before the onset of the reproductive phase is of marginal value for diagnosing defects of ovarian function, such as gonadal dysgenesis in Ullrich-Turner syndrome, agenesis, or hypogonadotropic hypogonadism. In contrast, elevated E2 in younger individuals is strongly indicative of precocious puberty or steroid hypersecretion (virilization or hyperandrogenism) (31). This underlines the importance of using adequate E2 reference values for evaluating children with precocious puberty (7,32).
PRL is a pleiotropic globular protein composed of 199 amino acids, is secreted via adenohypophysis in response to stimuli necessary for milk secretion, and exerts hormonal functions. This protein plays a crucial role in several physiological processes, and its quantification in both males and females has been associated with various diseases (33). Considering its profound clinical significance, we determined the RI for PRL based on our findings. Notably, since PRL levels increases in females at the age of 15, an additional RI was required for females only. There is some variability in the reported lower and upper reference limits between our research and other studies (4,7,9). The RIs of every partitioning group established with the Mindray CL-6000i were moderately narrower or wider than the previously reported RIs. For instance, the RI for PRL level in females 30 days to 12 months old was 3.97–70.99 µg/L on the Beckman Coulter device but 6.09–70.31 ng/mL for the CL-6000i (4). This variability can be attributed to differences in detection limits and precision of the assay, which depend on the analyzer used. Therefore, it is appropriate to establish method-specific RIs. These disparities underscore the importance of establishing RIs tailored to specific populations, as manufacturers often provide general intervals with a wide range based on their commercial validation studies.
TESTO is the primary male sex hormone secreted by the tests, ovaries, and adrenal glands. Therefore, measuring TESTO levels is crucial for accurately diagnosing various diseases, especially androgen deficiency in males (i.e., hypogonadism) and androgen excess in females (such as polycystic ovary syndrome and hirsutism) (34). Although some studies indicate that TESTO concentrations begin to decline within the first few days or weeks of life (1,9), these changes were not observed in our study. Additionally, the PROG levels remained consistent throughout childhood and increased during puberty. The clinical potential implications of progesterone in children primarily relate to its role in puberty, sexual development, hormonal balance, and stress response. These implications differ from those in adults or pregnant women and cannot be directly compared to specific research findings related to those populations. Therefore, it is important to consider the physiological context and age group when establishing the RI of PROG. Interestingly, six age-specific RIs for PROG were necessary to cover the age range from birth to 19 years in males, whereas four age partitions were required for females. Additionally, females undergo more significant changes in PROG levels during normal growth.
Dynamic and complex physiological patterns were observed for all sex hormones, underscoring the paramount importance of considering age and sex stratification when assessing pediatric endocrinopathies. Overall, the distribution trend of reference values were similar to those from the CALIPER cohort and other initiatives across various analytical platforms (7,15,35), underscoring the resilience and trustworthiness of the reported physiological trends. Although the detailed hormone profiles uncovered in this study are comparable to those documented in previous reports, conducting an absolute comparison between the estimated RIs and those documented in the literature poses challenges due to inconsistent partitioning methods (1,7,8). There are some differences between the RI data of this study and European and American children, suggesting that there are regional differences or ethnic differences in the RI of children’s clinical test indicators, and the research data of foreign children are not applicable to Chinese children. Overall, slight variations in the reported lower and upper reference limits were observed across various studies.
One of the strengths of our study lies in its comprehensive coverage of the age spectrum, from birth to 19 years, with participants being subdivided into age groups containing a substantial number of samples. However, this study was also subject to a number of limitations. First, as a single-center study, our cohort predominantly comprised individuals of Chinese descent. This demographic constraint potentially limits the applicability of our derived RIs to populations of other ethnicities. Second, we did not account for the influence of Tanner staging or body mass index (BMI) on the RIs of sex hormones among children aged 9–15 years, which is an area we intend to address in future research. Third, the diagnostic validity, sensitivity, and specificity of these RIs for endocrine disorders in clinical settings need to be further clarified and optimized.
Conclusions
We successfully established age- and sex-specific RIs for E2, FSH, LH, PRL, PROG, and TESTO on the Mindray CL-6000i analytical platform, enabling laboratories to use this system effectively. The application of these RIs facilitates accurate and prompt result interpretation for clinicians. However, as recommended by the CLSI C28-A3 guidelines (19), RIs should be validated for each clinical laboratory’s local pediatric population and individual analyzer before implementation.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-399/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-399/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-399/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-399/coif). W.L., L.L. and C.Q. are from Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted according to the Declaration of Helsinki (as revised in 2013) for research on human participants, and was approved by the institutional ethics board of Wuhan Children’s Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology (No. 2024R042-E01). Written informed consent was obtained from all participants (provided by at least one guardian).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adeli K, Higgins V, Trajcevski K, et al. The Canadian laboratory initiative on pediatric reference intervals: A CALIPER white paper. Crit Rev Clin Lab Sci 2017;54:358-413. [Crossref] [PubMed]
- Yang D, Su Z, Zhao M. Big data and reference intervals. Clin Chim Acta 2022;527:23-32. [Crossref] [PubMed]
- Konforte D, Shea JL, Kyriakopoulou L, et al. Complex biological pattern of fertility hormones in children and adolescents: a study of healthy children from the CALIPER cohort and establishment of pediatric reference intervals. Clin Chem 2013;59:1215-27. [Crossref] [PubMed]
- Karbasy K, Lin DC, Stoianov A, et al. Pediatric reference value distributions and covariate-stratified reference intervals for 29 endocrine and special chemistry biomarkers on the Beckman Coulter Immunoassay Systems: a CALIPER study of healthy community children. Clin Chem Lab Med 2016;54:643-57. [Crossref] [PubMed]
- Chulani VL, Gordon LP. Adolescent growth and development. Prim Care 2014;41:465-87. [Crossref] [PubMed]
- Spaziani M, Tarantino C, Tahani N, et al. Hypothalamo-Pituitary axis and puberty. Mol Cell Endocrinol 2021;520:111094. [Crossref] [PubMed]
- Elmlinger MW, Kühnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med 2002;40:1151-60. [Crossref] [PubMed]
- Schüring AN, Kelsch R, Pierściński G, et al. Establishing reference intervals for sex hormones on the analytical platforms Advia Centaur and Immulite 2000XP. Ann Lab Med 2016;36:55-9. [Crossref] [PubMed]
- Zec I, Kučak I, Begčević I, et al. Reference intervals for reproductive hormones in prepubertal children on the automated Roche cobas e 411 analyzer. Clin Biochem 2012;45:1206-12. [Crossref] [PubMed]
- Xiao L, Yang C, Gu W, et al. Associations between serum copper, zinc, selenium level and sex hormones among 6-19 years old children and adolescents in NHANES 2013-2016. Front Endocrinol (Lausanne) 2022;13:924338. [Crossref] [PubMed]
- Orçun A, Yildiz Z, Köroğlu Dağdelen L. Pediatric reference intervals for Free Testosterone, 17-OH Progesterone, Androstenedione, and IGF-1 with chemiluminescence immunoassay. Steroids 2022;186:109078. [Crossref] [PubMed]
- Klein DA, Emerick JE, Sylvester JE, et al. Disorders of Puberty: An Approach to Diagnosis and Management. Am Fam Physician 2017;96:590-9. [PubMed]
- Johannsen TH, Main KM, Ljubicic ML, et al. Sex Differences in Reproductive Hormones During Mini-Puberty in Infants With Normal and Disordered Sex Development. J Clin Endocrinol Metab 2018;103:3028-37. [Crossref] [PubMed]
- Kuijper EA, Ket JC, Caanen MR, et al. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 2013;27:33-63. [Crossref] [PubMed]
- Tahmasebi H, Asgari S, Hall A, et al. Influence of ethnicity on biochemical markers of health and disease in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med 2020;58:605-17. [Crossref] [PubMed]
- Chen HM, Chen CC, Chen JJ, et al. Reference intervals for thyroid hormone, sex hormone, and clinical biochemical tests in cord blood from Taiwanese newborn - TMICS cohort. Clin Chim Acta 2023;541:117247. [Crossref] [PubMed]
- Peng X, Peng Y, Zhang C, et al. Reference intervals of 14 biochemical markers for children and adolescence in China: the PRINCE study. Clin Chem Lab Med 2022;60:1627-39. [Crossref] [PubMed]
- Yu S, Qiu L, Liu M, et al. Establishing reference intervals for sex hormones and SHBG in apparently healthy Chinese adult men based on a multicenter study. Clin Chem Lab Med 2018;56:1152-60. [Crossref] [PubMed]
- CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline Third Edition, CLSI document EP28-A3c. Wayne, PA, Clinical and Láboratory Standards Institute:2008.
- Bohn MK, Wilson S, Schneider R, et al. Pediatric reference interval verification for 17 specialized immunoassays and cancer markers on the Abbott Alinity i system in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med 2023;61:123-32. [Crossref] [PubMed]
- Yao C, Wu M, Liu M, et al. Age- and sex-specific reference intervals for thyroid hormones in a Chinese pediatrics: a prospective observational study of 1,279 healthy children. Transl Pediatr 2021;10:2479-88. [Crossref] [PubMed]
- Biro FM, Pinney SM, Huang B, et al. Hormone changes in peripubertal girls. J Clin Endocrinol Metab 2014;99:3829-35. [Crossref] [PubMed]
- Jones GRD, Haeckel R, Loh TP, et al. Indirect methods for reference interval determination - review and recommendations. Clin Chem Lab Med 2018;57:20-9. [Crossref] [PubMed]
- Colantonio DA, Kyriakopoulou L, Chan MK, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 2012;58:854-68. [Crossref] [PubMed]
- Casteel CO, Singh G. Physiology, Gonadotropin-Releasing Hormone. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Gurdeep Singh declares no relevant financial relationships with ineligible companies.: StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.; 2024.
- Greaves RF, Pitkin J, Ho CS, et al. Hormone modeling in preterm neonates: establishment of pituitary and steroid hormone reference intervals. J Clin Endocrinol Metab 2015;100:1097-103. [Crossref] [PubMed]
- Dickerman Z, Prager-Lewis R, Laron Z. Response of plasma LH and FSH to synthetic LH-RH in children at various pubertal stages. Am J Dis Child 1976;130:634-8. [PubMed]
- Rosner W, Hankinson SE, Sluss PM, et al. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab 2013;98:1376-87. [Crossref] [PubMed]
- Di Meo A, Yazdanpanah M, Higgins V, et al. Highly sensitive tandem mass spectrometric measurement of serum estradiol without derivatization and pediatric reference intervals in children and adolescents. Clin Chem Lab Med 2023;61:1820-8. [Crossref] [PubMed]
- Won EJ, Yi A, Ko YJ, et al. Establishment of Korean Pediatric Reference Intervals for Estradiol using Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Clin Biochem 2023;113:52-8. [Crossref] [PubMed]
- Frederiksen H, Johannsen TH, Andersen SE, et al. Sex-specific Estrogen Levels and Reference Intervals from Infancy to Late Adulthood Determined by LC-MS/MS. J Clin Endocrinol Metab 2020;105:754-68. [Crossref] [PubMed]
- Kulle AE, Caliebe A, Lamprecht T, et al. New liquid chromatography tandem mass spectrometry reference data for estradiol show mini-puberty in both sexes and typical pre-pubertal and pubertal patterns. Eur J Endocrinol 2024;190:401-8. [Crossref] [PubMed]
- Moya-Salazar J, Cerda SP, Cañari B, et al. Reference intervals of the sex hormonal profile in healthy women: A retrospective single-center study in Peru. Heliyon 2022;8:e10592. [Crossref] [PubMed]
- Nassar GN, Leslie SW. Physiology, Testosterone. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Stephen Leslie declares no relevant financial relationships with ineligible companies.: StatPearls Publishing 35. Copyright © 2024, StatPearls Publishing LLC.; 2024.
- Bohn MK, Higgins V, Asgari S, et al. Paediatric reference intervals for 17 Roche cobas 8000 e602 immunoassays in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med 2019;57:1968-79. [Crossref] [PubMed]


