
Risk factors and risk prediction model for recurrence in medulloblastoma
Highlight box
Key findings
• In the present study, we have developed and validated a nomogram for the prognosis of recurrent medulloblastoma by integrating independent prognostic indicators.
What is known and what is new?
• Medulloblastoma has a poor prognosis, and establishing a predictive model to determine treatment intensity is the current focus.
• We used neutrophil-to-lymphocyte ratio (NLR) as a prognostic indicator for medulloblastoma for the first time and established a Nomogram prediction model including NLR.
What is the implication, and what should change now?
• Future treatment of medulloblastoma should be developed towards more accurate risk assessment and individualized diagnosis and treatment. The developed nomograms would be better to be validated with further multicenter cohort studies with larger sample sizes.
Introduction
Medulloblastoma is the most prevalent malignant brain tumor in the pediatric population, exhibiting a wide age range of onset from infancy to adulthood, with a peak incidence observed between 3 and 6 years of age (1,2). At present, the established primary therapeutic approach for medulloblastoma entails surgical intervention, followed by cranial-spinal irradiation (CSI) and a boost to the tumor bed. Notably, the 5-year disease control rate for patients with standard risk (SR) disease is approximately 80%, while patients with high risk (HR) disease exhibit a disease control rate of approximately 60–70% (3-5). The treatment strategy for medulloblastoma patients is stratified based on age. Patients who are older than 3 years are categorized into either SR or HR risk groups, taking into consideration factors such as residual tumor size after surgery, presence of tumor dissemination (M stage), and histology. The radiation fields and prescribed doses for patients in the SR and HR risk groups differ (6).
In the event of medulloblastoma relapse following CSI, even individuals initially classified as SR face a significantly unfavorable prognosis, with a 5-year survival rate of approximately 10% after relapse diagnosis (7). Therefore, it is evident that even minor adjustments to treatment protocols across various therapeutic approaches can yield favorable outcomes for patients through enhanced disease management or decreased long-term adverse effects.
The evaluation of relapse risk in medulloblastoma before initiating treatment holds significant importance in determining the appropriate treatment modalities and intensities. Consequently, the establishment of a predictive model to ascertain the likelihood of medulloblastoma relapse becomes imperative in aiding clinical decision-making, given that previous classification criteria have not incorporated molecular subtyping and immune markers. The objective of this study is to construct an enhanced risk prediction model for relapse in medulloblastoma by integrating molecular subtyping and straightforward immune markers, such as neutrophil-to-lymphocyte ratio (NLR), into a nomogram. This quantitative tool enables the prediction of specific prognostic indicators, including survival and relapse rates, by considering weighted prognostic factors (8,9). The intended outcome is to facilitate clinical decision-making in the context of medulloblastoma. We present this article in accordance with the TRIPOD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-392/rc).
Methods
Inclusion of patients
Data from 316 patients with medulloblastoma treated at Guangdong Sanjiu Brain Hospital between January 2003 and September 2020 were collected. The study was approved by the Guangdong Sanjiu Brain hospital Ethics Committee (No. 2022-010-032). Given the retrospective nature of the study, informed consent was waived from all subjects and their legal guardian(s) by Guangdong Sanjiu Brain Hospital. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013). All methods were carried out in accordance with relevant guidelines and regulations. Patients were included based on the following criteria: (I) newly diagnosed with medulloblastoma; (II) excluded other tumors or comorbidities; (III) blood samples collected within one week before radiotherapy; and (IV) complete follow-up data. Patients were excluded if they met any of the following criteria: (I) follow-up time after medulloblastoma diagnosis was less than 12 months; (II) incomplete records of blood test results before medulloblastoma diagnosis; and (III) a history of other malignant tumors. Finally, 273 patients were included in this study. Patients were divided into training set (n=165) and validation set (n=108) using seed setting in R.
Treatment methods
Upon admission, patients underwent routine preoperative examinations such as magnetic resonance imaging (MRI) and/or head computed tomography (CT), laboratory tests including blood routine, liver and kidney function, coagulation function, infectious diseases, etc. After excluding surgical contraindications, patients underwent tumor resection surgery to the extent safely feasible. Some patients developed postoperative hydrocephalus and underwent ventriculoperitoneal shunt or extra ventricular drainage surgery. After surgery, radiation therapy was initiated as soon as possible within 4 weeks depending on the patient’s physical recovery. Patients were categorized into risk groups based on preoperative conditions, with the SR group meeting all of the following conditions: age ≥3 years, pathological type excluding anaplastic, postoperative residual lesion within the imaging cross-sectional area <1.5 cm3, and no evidence of tumor dissemination on spinal MRI and cerebrospinal fluid examination. The high-risk group meets one of the following conditions: (I) age <3 years; (II) postoperative residual lesion ≥1.5 cm3 within the imaging cross-sectional area; (III) pathological type is large cell or anaplastic; (IV) evidence of tumor dissemination is found on imaging and/or cerebrospinal fluid cytology examination.
Patients were treated with adjuvant therapy within 4 weeks after surgery in the absence of abnormalities. Treatment was graded according to the patient’s risk. The patients in the SR group received craniospinal radiotherapy (23.4 Gy/13 fractions). The local boost dose was up to 54 Gy. Vincristine 1.5 mg/m2 was synchronized weekly. Concurrent chemoradiotherapy followed by 6 to 8 courses of adjuvant chemotherapy. Patients in the high-risk group received 36 Gy of craniospinal radiotherapy, completed 20 times, with concurrent radiotherapy receiving vincristine 1.5 mg/m2 per week. After the concurrent chemoradiotherapy was completed, the patients shall receive the adjuvant chemotherapy for more than 8 cycles.
Radiation therapy was performed using a linear accelerator (Varian Medical System, Palo Alto, CA, USA) with a photon energy of 6 MV. The patient was held in the supine position using a mask with a thermoplastic mask. A CT image of the treatment plan was obtained with a reconstruction layer thickness of 1.5 mm. The CT images acquired were registered in the iPlan 4.0 Treatment Planning System (BrainLAB, Heimstetten, Germany) with head-mounted MR and spinal cord mosaics MRI imaging to identify the tumor bed and its surrounding structures. The whole central target area includes brain tissue, spinal cord and spinal cord cavity from the cranial top to sacral 2–3 vertebral bodies. Local targets included residual lesions, tumor beds, and adjacent subclinical lesions.
Adjuvant chemotherapy regimens include cisplatin + estramustine (nimodipine) + vincristine, ifosfamide + etoposide + vincristine, cisplatin + cyclophosphamide + vincristine, and others.
Data collection
Baseline characteristics, including sex, age, Karnofsky performance score (KPS), histological classification, extent of surgical resection, tumor volume, presence of hydrocephalus, presence of intracranial and/or spinal cord dissemination, and molecular typing were extracted from the patient’s medical history. Blood samples were reviewed within 7 days prior to radiotherapy. The pre-radiotherapy NLR was calculated by serum neutrophil count/lymphocyte count. Some patients underwent genotyping by detecting gene variation and gene expression using second-generation gene sequencing technology on tumor tissues (10). Baseline treatment parameters including surgery-radiotherapy interval, total radiotherapy dose, and number of chemotherapy courses were also recorded.
Follow-up was performed with MRI of the brain and spinal cord every 3 months after treatment to identify recurrence or dissemination. The time to progression-free survival (PFS) was defined as the interval between the time of diagnosis and the time to recurrence of medulloblastoma. If no recurrence occurred, it was defined as the time from diagnosis to the last follow-up.
Statistical analysis
SPSS 21.0 (IBM, Chicago, Illinois, USA) was used for univariate and multivariate analysis, and R (4.0.3; https://www.r-project.org/) was used for survival analysis, Nomogram modeling and verification. Data were presented as counts and percentages or as medians and ranges or interquartile range (IQR). The optimal cutoff value for NLR was determined using X-Tile software (Yale X-Tile Bioinformatics Software, Version 3.6.1). Categorical variables were analyzed for survival using the Kaplan-Meier method and for significant differences between groups using the log-rank test. In the training cohort, cox proportional risk regression model was used for univariate and multivariate analyses. Single factor analysis was performed to identify important risk factors for PFS. Variables with p values less than 0.10 were further included in the multivariate Cox proportional risk regression model. The hazard ratio was expressed as a 95% confidence interval (CI). In the final multivariate analysis, variables with a P value <0.05 were considered to constitute independent prognostic factors for PFS.
Nomogram development and validation
Based on the results of multivariate cox analysis, the rms package in the R was used to develop nomograms predicting PFS at 48, 60, and 72 months in patients with medulloblastoma. Harrell’s Concordance Index (C-index) was used to evaluate the accuracy of the prediction. If the C-index is greater than 0.7 and the calibration curve is approximately consistent with the basic curve, the nomogram prognosis map (11) has good prognostic significance. The predicted nomogram was internally validated using calibration curves by 500–1,000 bootstrap re-sampling (12). We also quantified the identification performance of the nomograms using the area under the ROC curve. The total number of points for each patient was divided into two groups (low, high) based on the sum of the calculated nomogram and patient risk strata, with the sum of the risk scores in the 25th and 75th percentiles as the cutoff value. The Kaplan-Meier curve of high-risk group was drawn to further evaluate the correction effect.
Results
Baseline characteristics
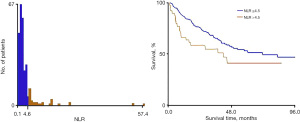
A retrospective analysis of 273 newly diagnosed patients with medulloblastoma was performed. Of these, 165 patients (approximately 60%) were randomized to the training cohort to construct nomogram, and the remaining 108 patients (approximately 40%) were assigned to the validation cohort. Baseline characteristics for the overall cohort are presented in Table 1. Out of the 273 patients, 108 experienced a recurrence, representing 39.6% of the total cases. Median follow-up was 30 and 27 months in the training and validation cohorts, respectively. The median PFS for the training and validation cohorts was 90 and 80 months, respectively. The optimal cutoff values of NLR calculated by X-tail software analysis were 4.5, as shown in Figure 1.
Table 1
| Clinical features | Full cohort (N=273) | Training cohort (N=165) | Validation cohort (N=108) |
|---|---|---|---|
| Median KPS | 70 [30–90] | 70 [30–90] | 70 [30–90] |
| Gender | |||
| Male | 139 (50.9) | 82 (49.7) | 57 (52.8) |
| Female | 134 (49.1) | 83 (50.3) | 51 (47.2) |
| Median age (years) | 8.6 [0.7–51] | 8.28 [0.7–41] | 8.7 [0.9–51] |
| Age (years) | |||
| <3 | 25 (9.2) | 15 (9.1) | 10 (9.3) |
| ≥3 | 248 (90.8) | 150 (90.9) | 98 (90.7) |
| Median TD size maximum diameter (mm) | 37 [22–69] | 37 [22–69] | 43 [22–67] |
| Tumor diameter (mm) | |||
| ≥38 | 101 (37.0) | 62 (37.6) | 39 (36.1) |
| <38 | 172 (63.0) | 103 (62.4) | 69 (63.9) |
| Genotyping or not | |||
| Yes | 90 (33.0) | 55 (33.3) | 35 (32.4) |
| No | 183 (67.0) | 110 (66.7) | 73 (67.6) |
| G3 group or not | |||
| Yes | 20 (7.3) | 11 (6.7) | 9 (8.3) |
| No | 253 (92.7) | 154 (93.3) | 99 (91.7) |
| NLR | |||
| ≥4.5 | 43 (15.7) | 29 (17.6) | 14 (13.0) |
| <4.5 | 220 (80.6) | 129 (78.2) | 91 (84.3) |
| NA | 10 (3.7) | 7 (4.2) | 3 (2.7) |
| Residual tumor (cm3) | |||
| <1.5 | 211 (77.3) | 129 (78.2) | 82 (75.9) |
| ≥1.5 | 62 (22.7) | 36 (21.8) | 26 (24.1) |
| Dissemination | |||
| No | 172 (63.0) | 99 (60.0) | 73 (67.6) |
| Yes | 101 (37.0) | 66 (40.0) | 35 (32.4) |
| Risk group | |||
| Standard risk | 132 (48.4) | 77 (46.7) | 55 (50.9) |
| High risk | 141 (51.6) | 88 (53.3) | 53 (49.1) |
| Hydrocephalus | |||
| Yes | 218 (79.9) | 130 (78.8) | 88 (81.5) |
| No | 42 (15.4) | 29 (17.6) | 13 (12) |
| NA | 13 (4.8) | 6 (3.6) | 7 (6.5) |
| Interval time radiotherapy interval | |||
| ≤4 weeks | 123 (45.05) | 70 (42.4) | 53 (49.1) |
| >4 weeks | 123 (45.05) | 77 (46.7) | 46 (42.6) |
| NA | 27 (9.9) | 18 (10.9) | 9 (8.3) |
Data are presented as median [range] or n (%). Residual tumor: residual tumor size post-operation; Interval time: interval time between surgery and radiotherapy. KPS, Karnofsky performance score; TD, tumor diameter, NLR, neutrophil-to-lymphocyte ratio; NA, not applicable.
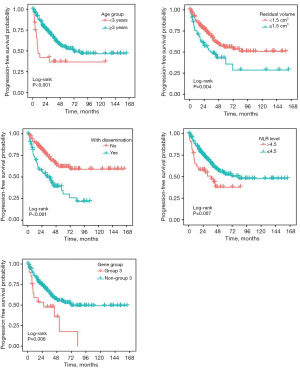
Univariate and multivariate cox regression analyses of brain metastases in the training cohort to determine the relationship between variables and PFS are described in Table 2. Univariate correlations were quantified as significant for age, sex, degree of surgical resection, pre-radiotherapy dissemination, pre-radiotherapy NLR, genotyping G3, surgery-radiotherapy interval of more than 4 weeks, and tumor diameter of more than 38 mm. In multivariate cox regression analysis, the five parameters were defined as the independent prognostic factors of patient recurrence, i.e., initial diagnosis age, residual degree after surgical resection, dissemination before radiotherapy, NLR before radiotherapy and classification as G3, as shown in Figure 2.
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Risk factor (95% CI) | P value | Risk factor (95% CI) | P value | ||
| Gender | |||||
| Male | 1 | 1 | |||
| Female | 1.075 (0.736–1.568) | 0.71 | 0.771 (0.503–1.183) | 0.23 | |
| Age (years) | |||||
| <3 | 1 | 1 | |||
| ≥3 | 0.398 (0.226–0.669) | 0.001 | 0.401 (0.216–0.743) | 0.004 | |
| Tumor diameter (mm) | |||||
| <38 | 1 | ||||
| ≥38 | 1.324 (0.879–1.994) | 0.18 | 1.265 (0.811–1.972) | 0.30 | |
| G3 group or not | |||||
| Yes | 1 | 1 | |||
| No | 0.448 (0.251–0.801) | 0.007 | 0.625 (0.328–1.192) | 0.050 | |
| NLR | |||||
| ≥4.5 | 1 | 1 | |||
| <4.5 | 0.533 (0.335–0.849) | 0.008 | 0.625 (0.328–0.991) | 0.046 | |
| Residual tumor (cm3) | |||||
| <1.5 | 1 | 1 | |||
| ≥1.5 | 1.817 (1.208–2.732) | 0.004 | 1.547 (0.959–2.496) | 0.07 | |
| Dissemination | |||||
| No | 1 | 1 | |||
| Yes | 2.491 (1.703–3.643) | 0.001 | 1.997 (1.307–3.052) | 0.003 | |
| Hydrocephalus | |||||
| Yes | 1 | 1 | |||
| No | 0.811 (0.480–1.368) | 0.43 | 0.908 (0.533-1.547) | 0.72 | |
| Interval time (weeks) | |||||
| ≤4 | 1 | 1 | |||
| >4 | 2.017 (1.508-2.697) | 0.001 | 1.713 (1.203–2.440) | 0.003 | |
Residual tumor: residual tumor size post-operation; Interval time: interval time between surgery and radiotherapy. NLR, neutrophil-to-lymphocyte ratio; CI, confidence interval.
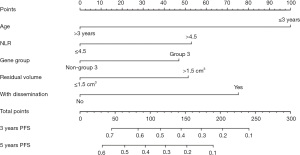
The establishment of nomogram
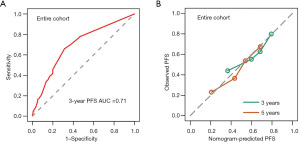
According to the results of multivariate cox regression analysis, age, gender, degree of surgical resection, dissemination before radiotherapy, NLR before radiotherapy, and genotyping whether G3 were independent prognostic factors, as shown in Table 2. These factors were integrated to form nomogram, as shown in Figure 3. The nomogram C-index for the predicted PFS in the training and validation cohorts was 0.749 and 0.736, respectively. The training cohort predicted a nomogram AUC of 0.723 for the 36-month OS and a validation cohort of 0.709, as shown in Figure 4.
Risk stratification for relapse
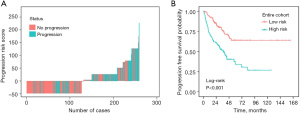
To further explore the predictive power of nomograms, we determined the total number of points per patient based on the nomograms throughout the cohort. We classified the patients as low- and high-risk according to the 50th percentile of the sum of the risk scores. Across the cohort, the Kaplan-Meier curve based on PFS showed significant differences (P<0.001) between the low- and high-risk groups predicted by nomogram, as shown in Figure 5.
Discussion
Medulloblastoma is a primitive neuroectodermal tumors (PNETs), which is sometimes called cerebellar PNETS because it is located in the cerebellum (13). Medulloblastomas are still classified as embryonal tumors in the 2021 edition of the World Health Organization (WHO) classification of central nervous system. Medulloblastomas can occur in all ages and account for about 1–2% of adult brain tumors, but they are the most common malignant brain tumors in childhood, with a slightly higher incidence in male (14,15). Medulloblastoma is located in the fourth ventricle, and its clinical feature is usually increased intracranial pressure. In addition, because of its metastatic tendency, about one-third of medulloblastomas will be disseminated in the central nervous system, and a small percentage of patients show extraneural metastasis (14). In terms of treatment, comprehensive treatment including surgery, radiotherapy, and chemotherapy is usually used for the treatment of medulloblastoma (16). At present, due to the improvement of surgical methods, non-surgical treatment and diagnostic imaging, about 70% of children with medulloblastoma can be cured (17). However, the PFS rate of high-risk children with medulloblastoma is less than 50%, and tumor recurrence remains an important cause of death (18-20). In this study, 273 children with medulloblastoma were analyzed. The 5-year PFS rate was 48%, and tumor recurrence remained the leading cause of death for medulloblastoma.
High-intensity chemotherapy and higher doses with greater radiation range (such as craniospinal radiotherapy) are applied to reduce recurrence. Many long-term survivors have severe neurocognitive impairment and other treatment-related toxicities due to high-intensity treatment. Therefore, it is still necessary to deeply understand the genesis and tumor stratification of medulloblastomas so that treatment strategies can be optimized for more rational individualized treatment in the future. Including pre-treatment dissemination, age, and tumor genotyping proven relevance to the prognosis of post-treatment medulloblastoma (21,22).
The recurrence of medulloblastoma is related not only to the extent of dissemination of the central nervous system disease at the time of diagnosis, the age at the time of diagnosis, the size of the residual lesion after surgery, and the tumor histopathology, but also to the biological/molecular characteristics of the tumor cells and the immune state of the patient. In this study, we confirmed that the extent of disease dissemination, age at diagnosis, size of residual lesion after surgery, tumor histopathology, classification as G3, pre-treatment NLR are important prognostic factors for relapse after treatment of medulloblastoma. In addition, we have developed and validated an effective nomogram to predict the recurrence rate of medulloblastoma. The nomograms established by combining these five independent prognostic clinicopathologic parameters showed good predictability when compared with classical prognostic indicators.
Medulloblastomas have four major histological subtypes: classical, fibrotic/nodular, large cell/anaplastic (LCA), and broadly nodular. In children, the histology of large intercellular degeneration has been proved to be an independent indicator of poor prognosis, and fibrous hyperplasia/nodular variation is considered to have a good prognosis (23,24). However, there are histological subtypes present in adults and children (25) in addition to different prognoses, and these differences may be related to the molecular heterogeneity of each histological subtype. Therefore, in this study, we did not include the histological subtypes in the analysis indicators. On the one hand, due to the in-depth research on molecular mechanisms and genes, the validity of the histological subtypes as prognostic indicators has become increasingly weak. On the other hand, due to the large time span of samples from patients in the study, it is not possible to obtain a clear histological subtype under the same condition.
In the past 10 years, with the deepening of research on the molecular mechanism and gene of medulloblastoma, medulloblastoma was divided into four categories in 2012, i.e., WNT type, SHH type, non-WNT non-SHH3 type, and G4 type (26,27). On the basis of this classification, 12 subtypes were evolved. A retrospective study of the previous 4 subtypes revealed that WNT type had the best prognosis and Group 3 had the worst. In our study, the second-generation lateral science technology was used to detect the gene and gene expression of medulloblastoma, which was classified into WNT and SHH types, Group 3 and Group 4 by mathematical algorithm. In previous reports, the prognosis of Group 3 is the worst, showing the lowest survival rate in all age groups: for example, the 10-year survival rate of Group 3 is 39% in infants and about 50% in children and adults. This is similar to the results in our study, in that PFS was significantly lower in patients with Group 3 than in patients not with Group 3 (including those with unclear subgroups) (27). Since large intercellular variant medulloblastomas are mostly associated with Group 3 infants, large intercellular variant medulloblastomas have been shown to be associated with prognosis in previous studies.
The immune system plays an important role in the pathogenesis of cancer and in response to immunotherapy. T-lymphocyte has been proved to be a key factor in the immune system’s response to cancer cells (20). Neutrophils and monocytes can promote tumor progression (28). The increased number of neutrophils in peripheral blood has been associated with poor prognosis in various cancers (29). Neutrophils, lymphocytes and monocytes are found to be of some value in predicting the prognosis of many common solid tumors (30). These patients with poor prognosis often find an increase in neutrophils and a decrease in lymphocytes in the circulatory system, thus leading to an increase in NLR (31). Michal and colleagues conducted a retrospective study of 120 pediatric brain cancer inpatients at Dana Children’s Hospital and Bruno Medical Center and 171 pediatric inpatients who were admitted at the same time. The NLR was calculated and the difference was determined to be statistically significant by paired t-test. NLR is significantly increased in pediatric patients with brain cancer. High NLR is the result of low lymphocyte and high neutrophil binding. Both of these factors may play a role in the development and reproduction of cancer. In previous studies on medulloblastoma, the absolute value of neutrophils in peripheral blood (32), the ratio of neutrophils to lymphocytes in peripheral blood (33) and the ratio of neutrophils to platelets in peripheral blood (33) were all used as measurement indexes to simply evaluate the immune function. In this study, the relatively simple index NLR was used, and it was also found that in the medulloblastoma population, a higher recurrence rate occurred when NLR >4.5, which is similar to the cutoff values in other studies (33,34).
There are some defects in this study. First, it was a single-center retrospective study. Although the training queue-based nomogram has been verified internally, it needs further verification externally to better ensure the effectiveness of the nomogram. Second, we classified some patients as unclassified because they were not subjected to molecular tests, which may have caused a statistical shift. Third, because treatment strategies for children under 3 years of age differ significantly from those for patients over 3 years of age, there may be a treatment baseline inconsistency.
Conclusions
In summary, in the present study, we have developed and validated an nomogram for the prognosis of recurrent medulloblastoma by integrating independent prognostic indicators. Future treatment of medulloblastoma should be developed towards more accurate risk assessment and individualized diagnosis and treatment. The developed nomograms would be better to be validated with further multicenter cohort studies with larger sample sizes.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-392/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-392/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-392/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-392/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Guangdong Sanjiu Brain hospital Ethics Committee
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Millard NE, De Braganca KC. Medulloblastoma. J Child Neurol 2016;31:1341-53. [Crossref] [PubMed]
- McGovern SL, Grosshans D, Mahajan A. Embryonal brain tumors. Cancer J 2014;20:397-402. [Crossref] [PubMed]
- Salloum R, Chen Y, Yasui Y, et al. Late Morbidity and Mortality Among Medulloblastoma Survivors Diagnosed Across Three Decades: A Report From the Childhood Cancer Survivor Study. J Clin Oncol 2019;37:731-40. [Crossref] [PubMed]
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 2006;24:4202-8. [Crossref] [PubMed]
- Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group Phase I/II study. J Clin Oncol 2012;30:2648-53. [Crossref] [PubMed]
- Srikantha U, Balasubramaniam A, Santosh V, et al. Recurrence in medulloblastoma - influence of clinical, histological and immunohistochemical factors. Br J Neurosurg 2010;24:280-8. [Crossref] [PubMed]
- Johnston DL, Keene D, Strother D, et al. Survival Following Tumor Recurrence in Children With Medulloblastoma. J Pediatr Hematol Oncol 2018;40:e159-63. [Crossref] [PubMed]
- Choi SH, Park SW, Seong J. A nomogram for predicting survival of patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Radiother Oncol 2018;129:340-6. [Crossref] [PubMed]
- Chisholm JC, Marandet J, Rey A, et al. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: a nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol 2011;29:1319-25. [Crossref] [PubMed]
- Liu KW, Pajtler KW, Worst BC, et al. Molecular mechanisms and therapeutic targets in pediatric brain tumors. Sci Signal 2017;10:eaaf7593. [Crossref] [PubMed]
- Cong R, Kong F, Ma J, et al. Combination of preoperative neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and monocyte-lymphocyte ratio: a superior prognostic factor of endometrial cancer. BMC Cancer 2020;20:464. [Crossref] [PubMed]
- Pan H, Shi X, Xiao D, et al. Nomogram prediction for the survival of the patients with small cell lung cancer. J Thorac Dis 2017;9:507-18. [Crossref] [PubMed]
- Saran F, Baumert BG, Creak AL, et al. Hypofractionated stereotactic radiotherapy in the management of recurrent or residual medulloblastoma/PNET. Pediatr Blood Cancer 2008;50:554-60. [Crossref] [PubMed]
- Bartlett F, Kortmann R, Saran F. Medulloblastoma. Clin Oncol (R Coll Radiol) 2013;25:36-45. [Crossref] [PubMed]
- Packer RJ, Vezina G. Management of and prognosis with medulloblastoma: therapy at a crossroads. Arch Neurol 2008;65:1419-24. [Crossref] [PubMed]
- Fan X, Eberhart CG. Medulloblastoma stem cells. J Clin Oncol 2008;26:2821-7. [Crossref] [PubMed]
- Leary SE, Olson JM. The molecular classification of medulloblastoma: driving the next generation clinical trials. Curr Opin Pediatr 2012;24:33-9. [Crossref] [PubMed]
- Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol 2004;5:209-18. [Crossref] [PubMed]
- Ellison DW, Clifford SC, Gajjar A, et al. What's new in neuro-oncology? Recent advances in medulloblastoma. Eur J Paediatr Neurol 2003;7:53-66. [Crossref] [PubMed]
- Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular Thommen DS, Schumacher TN. T Cell dysfunction in cancer. Cancer Cell 2018;33:547-62.
- Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol 1999;17:832-45. [Crossref] [PubMed]
- Yao MS, Mehta MP, Boyett JM, et al. The effect of M-stage on patterns of failure in posterior fossa primitive neuroectodermal tumors treated on CCG-921: a phase III study in a high-risk patient population. Int J Radiat Oncol Biol Phys 1997;38:469-76. [Crossref] [PubMed]
- Eberhart CG, Kepner JL, Goldthwaite PT, et al. Histopathologic grading of medulloblastomas: a Pediatric Oncology Group study. Cancer 2002;94:552-60. [Crossref] [PubMed]
- Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 2005;352:978-86. [Crossref] [PubMed]
- Brandes AA, Bartolotti M, Marucci G, et al. New perspectives in the treatment of adult medulloblastoma in the era of molecular oncology. Crit Rev Oncol Hematol 2015;94:348-59. [Crossref] [PubMed]
- Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 2012;123:465-72. [Crossref] [PubMed]
- Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 2012;123:473-84. [Crossref] [PubMed]
- Rosales C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol 2018;9:113. [Crossref] [PubMed]
- Schmidt H, Bastholt L, Geertsen P, et al. Elevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic model. Br J Cancer 2005;93:273-8. [Crossref] [PubMed]
- Leitch EF, Chakrabarti M, Crozier JE, et al. Comparison of the prognostic value of selected markers of the systemic inflammatory response in patients with colorectal cancer. Br J Cancer 2007;97:1266-70. [Crossref] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Grassberger C, Shinnick D, Yeap BY, et al. Circulating Lymphocyte Counts Early During Radiation Therapy Are Associated With Recurrence in Pediatric Medulloblastoma. Int J Radiat Oncol Biol Phys 2021;110:1044-52. [Crossref] [PubMed]
- Li K, Duan WC, Zhao HB, et al. Preoperative Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio are Associated with the Prognosis of Group 3 and Group 4 Medulloblastoma. Sci Rep 2019;9:13239. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]


