
Association of testicular calcifications and intracranial germinoma: a case report and review of the literature
Highlight box
Key findings
• An 11-year-old adolescent male was diagnosed with an intracranial germ cell tumour and brain biopsy revealed a rare seminoma followed by multiple calcifications in both testes. Intracranial seminomas are rarely reported and testicular calcifications may be a potential causative factor.
What is known and what is new?
• Testicular calcifications and testicular microlithiasis have been identified as independent risk factors for testicular cancer (TC) in numerous previous observational studies and randomised controlled trials. And the main pathological type of TC is seminoma.
• Here we found a rare case of intracranial germ cell tumour, the specific pathological type of which is seminoma. Subsequent ultrasound revealed multiple calcifications in the testes.
What is the implication, and what should change now?
• Testicular calcifications are not only a high-risk factor for TC, but may also be a high-risk factor for intracranial germ cell tumours. In adolescent males aged 10–20 years, the presence of testicular calcifications should raise suspicion for intracranial lesions. In patients with coexisting intracranial disorders, brain biopsy should be performed as soon as possible to determine the pathological type before deciding on a treatment regimen.
Introduction
Seminomas and nonseminomas are the two main clinical subtypes of germ cell tumors (GCTs), which have significant clinical and prognostic implications. While nonseminomas are more likely to have metastatic disease and mixed GCTs, which are composed of two or more distinct GCT components, seminomas typically have a good prognosis (1). Among these, metastases from testicular cancers (TCs) and GCTs to the brain and spinal cord are uncommon, and metastases from seminomas are extremely rare (2). Testicular microlithiasis (TM) has been linked to testicular tumors in recent years, and this finding suggests that TM may be a risk factor for the development of testicular tumors (3-6). Additionally, because testicular microcalculias have been linked to both unclassified intratubular germ cell neoplasia (ITGCNU) and testicular germ cell tumors (TGCCTs), interest in these conditions has grown in recent years (7). Ultrasound surveillance is advised for patients with TM due to the connection with TC (8). In this case, a patient with a seminoma detected by an intracranially directed biopsy was found to have TM during hospitalisation, which may be a high-risk factor for intracranial seminoma. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-403/rc).
Case presentation
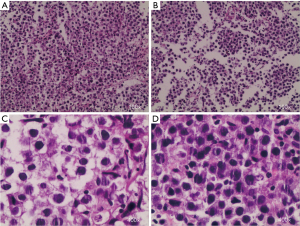
Here we report an 11-year-old male who had a headache for no apparent reason half a year ago, which was paroxysmal and relieved with rest, occasionally accompanied by left lower limb twitching, which usually lasted for about half a minute and was relieved. One month ago, he developed left limb weakness, with obvious symptoms on the left upper limb, and there was no walking impairment, and he was treated in a local hospital, and the cranial magnetic resonance (MR) examination showed that the abnormal signal foci in the right radial crown and basal ganglia area were considered to be tumor lesions. Later, the patient came to our hospital for further treatment, and the magnetic resonance angiography (MRA) was checked: there was no clear abnormality in the shape and size of the skull. The right basal ganglia area-radiation crown showed irregularly shaped and uneven slightly longer T1 slightly longer T2 mass shadow, the range was about 44 mm × 35 mm, and the nodular nodule in the lesion was enhanced and strengthened, the size of the nodule was about 5 mm × 4 mm, and the diffusion weighted imaging (DWI) showed an equal signal. The midline structure was slightly displaced to the left, and the right lateral ventricle was compressed. MRA: the right middle cerebral artery was compressed, and the bilateral internal carotid arteries, anterior cerebral arteries, basilar arteries, posterior cerebral arteries and their large branches ran normally, with smooth contours, and no obvious stenosis or occlusion was observed. Diffusion tensor imaging (DTI): right basal ganglia-radial crown white matter fiber bundles are sparse and partially interrupted. Perfusion weighted imaging (PWI): uneven perfusion of the right basal ganglia-radiation coronavirus. Magnetic resonance spectroscopy (MRS): increased Cho peak and decreased N-acetyl-L-aspartic acid (NAA) peak in right basal ganglia-radiation coronal lesions (Figure 1). After a comprehensive preoperative examination, considering the age of the patient and the possibility of malignancy of the disease, we first performed a right basal ganglia biopsy under robotic guidance and navigation, and the postoperative pathological results showed that the tumor cells with consistent morphology were diffuse and flaky under the microscope, the cytoplasm of the cells was rich, lightly stained or translucent, and the nucleolus and interstitial lymphocyte infiltration were visible, combined with immunohistochemistry, which was consistent with seminoma (Figure 2). After obtaining the pathological results, we did an ultrasound of the scrotum to rule out the risk of metastasis, and the ultrasound results showed that the size and shape of the bilateral testes were normal, the surface was smooth, the internal echo of the testicles was uneven, and scattered strong echo points could be seen, which was initially considered to be testicular calcifications (Figure 3). After the diagnosis of seminoma, the patient was transferred to the Department of Internal Medicine and underwent 5 rounds of chemotherapy and 3 times of radiotherapy, and the tumor shrank significantly after MR examination, and the patient achieved tumor remission and was discharged (Figure 4).



All procedures performed in this study were in accordance with the ethical standards of the ethics committee of The Third Affiliated Hospital of Sun Yat-sen University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the legal guardian(s) of the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
TM is an uncommon discovery on ultrasound examinations, with an incidence in the literature ranging from 0.7% to 6%. Multiple studies have emphasized the potential link between it and a higher likelihood of developing TC (9-12). Research has shown that when there are no risk factors present, the occurrence of TC in patients with TM is comparable to that of the whole population. Nevertheless, in the event that TM is detected and there are accompanying risk factors such as a personal history of TC, testicular atrophy, infertility, or cryptorchidism, the likelihood of cancer is significantly heightened (6). Experts recommend conducting a testicular biopsy to eliminate the possibility of carcinoma in situ (CIS) in males with TM and with one or more of the aforementioned risk factors (13). Several studies have discovered a notable correlation between TM and TCs (14), and malignancy is exclusively seen in teenage males (11), in the pediatric population, it is recommended to perform regular self-examinations and annual examinations in the absence of risk factors (15), during the stages of infancy, youth, and adolescence, this diagnostic method is particularly essential for evaluating typical gonadal development and identifying potential disorders (16). Ultrasound surveillance is unnecessary in the general population when TM is the sole anomaly among the clinical risk factors associated with the development of GCT (17). Recent investigations have found that the occurrence of TM is linked to a significantly greater rate of TC, approximately 18 times more prevalent, not just in children but also in men who are unable to conceive. A longitudinal investigation is required to ascertain whether this cross-sectional correlation truly indicates the increased vulnerability of TM infertile men to develop TC in the long run (18). Recent research has discovered that men who have testicular microcalculi and testicular atrophy are more likely to develop germ cell neoplasia in situ. A study conducted by Danish researchers discovered that for every patient diagnosed with testicular microcalculias and who underwent testicular biopsies at three Danish hospitals between 2007 and 2021, orthotopic germ cell neoplasia was found in 13 out of 167 cases. Eleven of these individuals experienced testicular atrophy, which led to a higher level of illness in this group compared to other risk factors. Furthermore, it is advisable to contemplate performing a biopsy in males exhibiting subfertility and bilateral testicular microstones (12). Pure seminoma is a specific kind of TC that makes up about 50% of all TGCCTs. The metastatic rate to the central nervous system is quite low (19). So far, in the Web of Science search, we have found only one case (20). This GCT, which is found in the central nervous system, represents less than 4% of cancers placed inside the skull and primarily impacts children and young adults. Typically, they can be found in the pineal gland and suprasellar areas. The predominant histologic subtype is GCT, accounting for 65% of cases, and it is associated with the most favorable prognosis (21). Radiotherapy is a successful treatment option for patients with extragonadal seminoma and typically yields positive results (22). In this group of children, seminoma was detected at the same time as intracranial biopsy, which may be a high-risk factor for the development of the disease, and timely biopsy and color ultrasound screening may be a feasible way to diagnose and treat such patients.
Conclusions
Intracranial germinoma has been reported rarely, and its pathogenesis remains to be explored. Through a literature review, we found that our patient also had features of TM. For adolescent patients, a complete biopsy and color ultrasound may be a necessary option.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-403/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-403/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-403/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the ethics committee of the Third Affiliated Hospital of Sun Yat-sen University and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the legal guardian(s) of the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sadiq Q, Khan FA. Germ Cell Seminoma. Treasure Island (FL): StatPearls Publishing; 2025.
- Yumioka T, Morizane S, Makishima K, et al. Brain and spinal cord metastases with seminoma: A case report. IJU Case Rep 2022;5:464-8. [Crossref] [PubMed]
- Betancourt Sevilla MD, Granda González DF. Association between testicular cancer and microlithiasis. Actas Urol Esp (Engl Ed) 2022;46:587-99. [Crossref] [PubMed]
- Pedersen MR, Rafaelsen SR, Møller H, et al. Testicular microlithiasis and testicular cancer: review of the literature. Int Urol Nephrol 2016;48:1079-86. [Crossref] [PubMed]
- 't Hoen LA, Bhatt NR, Radmayr C, et al. The prognostic value of testicular microlithiasis as an incidental finding for the risk of testicular malignancy in children and the adult population: A systematic review. On behalf of the EAU pediatric urology guidelines panel. J Pediatr Urol 2021;17:815-31. [Crossref] [PubMed]
- Leblanc L, Lagrange F, Lecoanet P, et al. Testicular microlithiasis and testicular tumor: a review of the literature. Basic Clin Androl 2018;28:8. [Crossref] [PubMed]
- Tan MH, Eng C. Testicular microlithiasis: recent advances in understanding and management. Nat Rev Urol 2011;8:153-63. [Crossref] [PubMed]
- Winter TC, Kim B, Lowrance WT, et al. Testicular Microlithiasis: What Should You Recommend? AJR Am J Roentgenol 2016;206:1164-9. [Crossref] [PubMed]
- Catanzariti F, Cantoro U, Lacetera V, et al. Testicular microlithiasis and dyspermia: is there any correlation? Arch Ital Urol Androl 2014;86:20-2. [Crossref] [PubMed]
- Wang T, Liu L, Luo J, et al. A Meta-Analysis of the Relationship between Testicular Microlithiasis and Incidence of Testicular Cancer. Urol J 2015;12:2057-64.
- Cooper ML, Kaefer M, Fan R, et al. Testicular microlithiasis in children and associated testicular cancer. Radiology 2014;270:857-63. [Crossref] [PubMed]
- Frandsen RH, Durukan E, von Rohden E, et al. Testicular biopsies in men with testicular microlithiasis and additional risk factors for cancer: A case series. Andrology 2024;12:1764-70. [Crossref] [PubMed]
- Fode M, Giwercman A, Bisbjerg R, Sønksen J. The significance of testicular microlithiasis. Ugeskr Laeger 2014;176:V02130145.
- Trout AT, Chow J, McNamara ER, et al. Association between Testicular Microlithiasis and Testicular Neoplasia: Large Multicenter Study in a Pediatric Population. Radiology 2017;285:576-83. [Crossref] [PubMed]
- Yesil S, Tanyildiz HG, Sahin G. How should we monitor boys with testicular microlithiasis? Pediatr Hematol Oncol 2016;33:171-7. [Crossref] [PubMed]
- Spaziani M, Lecis C, Tarantino C, et al. The role of scrotal ultrasonography from infancy to puberty. Andrology 2021;9:1306-21. [Crossref] [PubMed]
- Patel KV, Navaratne S, Bartlett E, et al. Testicular Microlithiasis: Is Sonographic Surveillance Necessary? Single Centre 14 Year Experience in 442 Patients with Testicular Microlithiasis. Ultraschall Med 2016;37:68-73. [Crossref] [PubMed]
- Barbonetti A, Martorella A, Minaldi E, et al. Testicular Cancer in Infertile Men With and Without Testicular Microlithiasis: A Systematic Review and Meta-Analysis of Case-Control Studies. Front Endocrinol (Lausanne) 2019;10:164. [Crossref] [PubMed]
- Sinicrope KD, Kaplan AB, Brastianos PK. Seminoma with Neoplastic Meningitis Treated with Craniospinal Irradiation. Oncologist 2018;23:1385-7. [Crossref] [PubMed]
- Hafiz MA, Ekanem IO. Cytologic diagnosis of seminoma metastatic to the central nervous system. A case report. Acta Cytol 1983;27:663-5.
- Losa F, García del Muro J, Germà JR. Primary germ cell tumors of the central nervous system. Neurologia 1997;12:249-54.
- Kersh CR, Constable WC, Hahn SS, et al. Primary malignant extragonadal germ cell tumors. An analysis of the effect of the effect of radiotherapy. Cancer 1990;65:2681-5. [Crossref] [PubMed]


