
Clinical characteristics of infants with protracted bacterial bronchitis: an observational cohort study
Highlight box
Key findings
• For infants, wheezing was an important and common clinical performance when they suffered from protracted bacterial bronchitis (PBB).
• The most common pathogens were Moraxella bacteria and Streptococcus pneumoniae.
• The most prominent chest computed tomography (CT) performance of PBB was uneven inflation in infants.
What is known and what is new?
• PBB is a common cause of persistent, wet cough in pre-school children. Several studies document Haemophilus influenzae as the most common bacteria found in the bronchoalveolar lavage (BAL) of subjects with PBB (47–81%), Streptococcus pneumoniae (24–39%) and Moraxella catarrhalis (19–43%) follow with variable percentages among different studies.
• In infants, wheezing is a symptom that cannot be ignored in addition to wet cough. Moraxella catarrhalis is of more significance than it is usually anticipated as an important pathogen of PBB.
What is the implication, and what should change now?
• Wheezing, as the main symptom of infant PBB, should be paid attention to. Wheezing is not only a manifestation of viral infection, but also bacterial infection in the airway.
• The pathogen spectrum of PBB in infants is slightly different from that in children, Moraxella catarrhalis is the most common pathogen, clinicians’ emphasis should be placed on the selection and course of antibiotics.
Introduction
Protracted bacterial bronchitis (PBB) is acknowledged as a significant contributor to chronic cough in pediatric populations (1,2). Since its initial identification as a separate diagnostic category in 2006 (3), PBB has been integrated into cough management guidelines and educational materials across many countries. It is caused by chronic endobronchial infection with organisms such as Haemophilus influenzae, Streptococcus pneumoniae, Klebsiella pneumoniae and Moraxella catarrhalis (4,5). The original diagnostic criteria for PBB included wet cough for four weeks, identifiable lower airway bacterial infection on bronchoalveolar lavage (BAL) culture, and response to antibiotics (amoxicillin/clavulanate) with resolution of cough within 2 weeks (6). The previous studies found that the diagnostic categories for chronic cough in children are heterogeneous and that the most common diagnosis was PBB (3,7,8). It is important to distinguish possible ongoing PBB from persistent cough following a viral infection or several successive viral infections with wet cough (acute bronchitis) (9,10). Due to similar clinical manifestations, the use of BAL was particularly helpful because it pinpointed the diagnosis and pathogens that are crucial in PBB (7,11). PBB is globally acknowledged as a major pediatric disease that contributes significantly to the overall burden of illness in children (1).
It is established that post-bronchiolitis bronchial hyperreactivity (PBB) is a prevalent etiology of chronic wet cough in preschool-aged children, specifically those between zero to six years old, on a global scale, although it can occasionally manifest in older individuals as well. The condition is diagnosed in approximately 11–41% of pediatric patients who seek consultation from a pulmonary specialist (12). Current research on PBB has predominantly overlooked the infant population, with some scholars contending that the concept of PBB in infants remains inadequately defined. Additionally, there are calls for more comprehensive investigations into this area. Due to the infrequent expectoration of sputum by children diagnosed with PBB, the predominant technique employed for sampling the lower respiratory tract is BAL, which is performed during flexible bronchoscopy (FB-BAL) (13). In our study of 37 infants with PBB, we, thus, provide more extensive clinical, laboratory, image, and BAL characterization of PBB in infants. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-509/rc).
Methods
Study participants
This was a prospective, observational study. Two hundred and eight patients with PBB on admission who attended Guangzhou Women and Children’s Medical Center from January 1, 2021, to January 1, 2024, were eligible for this study.
The inclusion criteria for the study were as follows:
- 28 days < age ≤1 year.
- PBB was defined as (i) presence of continuous, chronic (>4 weeks duration) wet or productive cough; (ii) absence of symptoms or signs (i.e., specific cough pointers) suggestive of other causes of wet or productive cough; and (iii) cough resolved following a two-week course of an appropriate oral antibiotic. Recurrent PBB was defined as >3 PBB episodes within 12 months.
- Informed consent was signed by the guardians of study participants.
Exclusion criteria for the study were as follows: individuals with known or suspected active tuberculosis; those with severe concomitant conditions, including chronic pulmonary diseases such as asthma, significant cardiovascular diseases, neoplasms, and renal or hepatic disorders; and individuals in an immunocompromised state, which encompasses primary immunodeficiency, acquired immunodeficiency syndrome, and the use of immunosuppressive medications prior to admission. Clinical information of children with severe adenovirus pneumonia was collected during the study period. The follow-up of the study participants was conducted again at one, three, six and twelve months after diagnosis to collect clinical information, including lower respiratory tract infections (LRTIs).
The protocol of this study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center of Guangzhou Medical University (No. 2021278B00). Informed consent was obtained from the patients’ parents or legal guardians.
Etiology
Pathogen detection was performed at the central hospital diagnostic laboratories on blood, throat swab, sputum and BAL samples. Bacterial and fungal species were cultured in Bactec 9120 auto microbial culturing hood (BD, New Jersey, USA) for 16–18 hours and characterized by VITEK Compact System (bioMérieux Diagnostics, Suzhou, China). Viral pathogens were detected either or both by Taqman qPCR (14) and Pneumoslide IgM ELISA kit for Mastadenovirus (ADV), Bocavirus (BOV), influenza virus A & B (IAV, IBV), respiratory syncytial virus (RSV), Enterovirus (EV), parainfluenza virus (PIV), Rhinovirus (RHV), Mycoplasma pneumoniae (MP) and Chlamydia pneumoniae (CP) (Vircell, Victoria Gastz, Spain).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA). Because continuous variables of this study were skewed in distribution, medians with interquartile range (IQR) were used to express the summarized data.
Results
Demographics of the cohort
In the present study, 37 infants with newly diagnosed PBB were included. According to the admission criteria, there were 30 boys (81.08%) and 7 girls (18.92%) with a median onset age of 6 months (IQR, 4–7.5 months). The median interval from onset to diagnosis was 2.5 months (IQR, 1.5–3.5 months). All patients were discharged after a median hospitalization period of 11 days (IQR, 8–16 days). The most common clinical presentations of PBB in infants were wet cough (37/37, 100.00%), wheezing (34/37, 91.89%), stridor (24/37, 64.86%) and tachypnea (10/37, 27.03%). The median follow-up was 12 months (IQR: 9–12 months). None of the patients in this cohort died. Before diagnosis, all patients received inhaled bronchodilator and corticosteroid therapy to alleviate wheezing and stridor (37/37, 100.00%). However, all infants exhibited a poor response to the treatment (37/37, 100.00%). The clinical features of patients are shown in Table 1.
Table 1
| Variable | Value |
|---|---|
| Demographic | |
| Age of onset (months) | 6 (4–7.5) |
| Age of diagnosis (months) | 10 (7–12) |
| Gender, male | 30 (81.08) |
| The interval from onset to diagnosis (months) | 2.5 (1.5–3.5) |
| Signs and symptoms | |
| Fever | 0 (0.00) |
| Wet cough | 37 (100.00) |
| Wheezing | 34 (91.89) |
| Stridor | 24 (64.86) |
| Tachypnea | 10 (27.03) |
| Moist crackles | 6 (16.22) |
| Runny nose | 2 (5.41) |
| Primary diseases | |
| Malnutrition | 6 (16.22) |
| Eczema | 10 (27.03) |
| Preterm birth | 2 (5.41) |
| Congenital heart disease | 0 (0.00) |
| Laboratory findings† | |
| White blood cells (×109/L) (ref: 5–12) | 11.00 (8.40–13.15) |
| Neutrophil (×109/L) (ref: 5–12) | 3.36 (2.76–4.08) |
| Hemoglobin (g/L) (ref: 105–145) | 123 (112.5–127) |
| Platelet (×1012/L) (ref: 140–440) | 426 (358–498.5) |
| HsCRP (mg/L) (ref: <5) | 0.85 (0.5–2.07) |
| PCT (ng/mL) (ref: <0.25) | 0.1 (0.1–0.11) |
| ESR (mm/h) (ref: 0–20) | 2 (2–2) |
Data are presented as median (IQR) or n (%). †, the data of immunoassays findings were collected from patients at admission. ESR, erythrocyto sedimentation rate; HsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; PBB, protracted bacterial bronchitis; PCT, procalcitonin.
Immunology detection
The immunologic detection and genetic tests at the time of diagnosis are shown in Table 2. There were 26 patients tested with immunoglobulin and complements at the beginning of the disease (26/37, 70.27%). Among them, only a few patients had reduced immunoglobulin (mainly IgA). There were another five patients who showed higher levels of IgE (5/26, 19.23%). Low complement C3 levels were found in 12 cases (12/26, 46.15%), and 3 cases exhibited low complement C4 levels (3/26, 11.54%). Eleven patients were detected with lymphocyte count. Only one case presented slightly decreasing CD4+ T lymphocyte counts (9.09%). The counts of other lymphocyte subsets were in the normal range in patients. All above abnormity indexes returned to normal after treatment.
Table 2
| Immunoassays findings | N (%) |
|---|---|
| Immunoglobulin (N=26) | |
| IgG decrease | 1 (3.85) |
| IgA decrease | 3 (11.54) |
| IgM decrease | 0 (0.00) |
| IgE increase | 5 (19.23) |
| Complements (N=26) | |
| C3 decrease | 12 (46.15) |
| C4 decrease | 3 (11.54) |
| Lymphocyte counts (N=11) | |
| CD4+ subsets decrease | 1 (9.09) |
| CD8+ subsets decrease | 0 (0.00) |
| CD19+ subsets decrease | 0 (0.00) |
| NK cells decrease | 0 (0.00) |
†, the data of immunoassays findings were collected from patients at admission. Ig, immunoglobulin; NK cell, nature killer cell; PBB, protracted bacterial bronchitis.
Etiology
Sputum culture was performed in 13 children [>25 neutrophils and <10 epithelial cells per low-power field (LPF) on Gram stain] (13/37, 35.14%) and BAL culture was performed in all children [≥104 colony-forming units (CFU)/mL] (37/37, 100.00%) to search for a causative agent (Figure 1). There were six patients with positive sputum cultures (6/13, 46.15%) and 32 patients with positive results from BAL (32/37, 86.49%).
At least one known pathogen was detected in 86.49% (32/37) of the patients, and six patients were identified as mixed infections (16.22%). Moraxella bacteria (12/37, 32.43%) and Streptococcus pneumoniae (11/37, 29.73%) were the most often diagnosed pathogens, followed by Stenotrophomonas maltophilia (7/37, 18.92%), Hemophilus influenzae (7/37, 16.22%), Staphylococcus aureus (7/37, 13.51%) and Klebsiella pneumonia (1/37, 2.70%). There were nine patients detected with multiple drug-resistant strains by BAL cultures (9/32, 28.13%). Streptococcus pneumoniae was found to be the most common multidrug resistant pathogens (7/9, 77.78%), next to Hemophilus influenzae (2/9, 22.22%).
In addition to bacterial cultures, a total of 10 children in this study tested positive for respiratory viruses using Taqman qPCR, including RSV (3/10, 30.0%), RHV (6/10, 60.0%), and human metapneumovirus (1/10, 10.0%).
Imaging and bronchoscopy findings
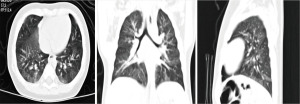
All cases were given chest X-ray during the disease, all showed bronchitis (37/37, 100.00%). All patients underwent chest CT scans because of recurrent or continuous cough and wheezing at diagnosis, all children demonstrated imaging abnormalities to differing extents (37/37, 100.00%). Uneven lung inflation was the most common form of chest CT manifestation in infants with PBB (27/37, 72.97%), which showed air trapping in some lung segments (Figure 2). Bronchitis (7/37, 18.92%) and local emphysema (3/37, 8.11%) were in second place.
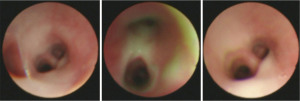
Meanwhile, FB-BAL was performed as an additional diagnostic test for all infants (37/37, 100.00%). Under bronchoscopy, thick pale yellow or yellow mucus was found to be attached to airway in all patients (Figure 3). Furthermore, tracheomalacia was observed in 11 patients (11/37, 29.73%).
Treatment and prognosis
The use of antibiotics was permitted for all infants with PBB after a definite diagnosis (37/37, 100.00%). Before diagnosis, all patients were also given inhaled hormone and bronchodilator therapy (37/37, 100.00%). However, all infants exhibited a poor response to the treatment (37/37, 100.00%). Empirical antibiotics were taken in 27 patients before admission (27/37, 72.97%). According to the antibiogram (ATB) sensitivity and resistance rate for drug-resistant strains combined with the clinical therapeutic effects, 19 infants adjusted the antibiotic treatment (19/37, 51.35%), and 18 infants continued the initial treatment (18/37, 48.65%). The type and dose of antibiotics used were shown in the supplementary table (https://cdn.amegroups.cn/static/public/tp-2024-509-1.xlsx).
In this study, cephalosporins (22/37, 59.46%) and penicillin antibiotics (21/37, 56.75%) have been selected more frequently in infants with PBB. Only three patients were treated with both antibiotics (3/37, 32.43%). The median course of antibiotics was 18 days (IQR, 14–24 days). All patients were followed up after discharge, the condition can be completely mitigated (34/37, 91.89%), only three patients developed recurrent PBB.
Discussion
PBB is defined by a persistent chronic wet or productive cough that occurs in the absence of identifiable alternative etiologies. This condition typically demonstrates a favorable response to a course of appropriate oral antibiotics administered for a duration of two weeks (15). The term has been acknowledged in clinical guidelines as a contributing factor to chronic wet cough in pediatric populations (16,17). PBB is a disease that exhibits a significant prevalence in certain contexts; a recent investigation indicated a prevalence rate of 10% for PBB within an Aboriginal community (18), and another study reported that up to 37.9% of children referred to tertiary care for chronic cough had PBB (19). PBB can be seen at any age in children but the incidence is higher in younger children (mean age 1.8–4.8 years) (20), and is more commonly seen in male children attending daycare (21). However, there are limited data on infants aged less than one year diagnosed with PBB. We prospectively analyzed the clinical data of 37 infants aged less than one year with PBB, including the clinical features, immunologic characteristics, pathogens, imaging findings and treatments. We found that wheezing was considerable in infants with PBB, and Moraxella bacteria and Streptococcus pneumoniae were the most frequently isolated pathogens. In particular, the most prominent CT imaging performance of PBB was uneven lung inflation in infants.
Presence of chronic wet cough has long been a classic symptom of PBB (22). Longitudinal studies indicated that around 60% of children diagnosed with PBB exhibited symptoms of wheezing (23), and another study shows a high proportion of caregivers reported “wheeze ever” in their child with PBB (74%) (21). Saglani et al. reported that positive bacterial growth was obtained in 12 out of 44 (27%) young children with severe recurrent wheeze, and the patients in this predominantly infected diagnostic category were significantly younger (24). Therefore, severe recurrent wheezing may indicate a bacterial infection in the airway of younger children. For infants with PBB, wheezing was the important symptom in our study, therefore PBB with chronic cough and wheezing was prone to misdiagnosis of asthma when the infants co-occurred with allergic diseases. Due to their young age, most of the children did not exhibit symptoms of allergic diseases, and only ten children had a history of eczema. The point of identification is that these infants usually show no response or low response to inhaling bronchodilator and corticosteroid. In our study, all patients were administered inhaled bronchodilators and corticosteroids throughout the treatment course; however, the responses were suboptimal. Pediatricians should be alert to the infants who present consistent wheezing and are ineffective to corticosteroids, they might suffer from PBB.
Children diagnosed with PBB typically do not exhibit immunodeficiencies (21). The previous research suggested that children with PBB typically appear well (20). They have normal growth and development and lack signs of underlying chronic suppurative lung disease (CSLD), such as digital clubbing, chest wall deformity, and adventitial auscultatory chest findings (21). Consequently, most of these children demonstrate normal serum immunoglobulin levels-specifically immunoglobulin (Ig) G, IgA, IgM, and IgE-by the time they reach a certain age. Additionally, they show a normal antibody-mediated response to protein antigens, such as tetanus, as well as to conjugated protein polysaccharide (12). In our study, their levels of immunoglobulins and lymphocyte subsets were almost normal or with a slight decline, all of them returned to normal after treatment. The immune mechanisms in PBB involve a combination of impaired mucociliary clearance, elevated antimicrobial peptide levels, neutrophil recruitment, and adaptive immune responses. One of the key factors in PBB is impaired mucociliary clearance, which prevents the effective removal of mucus and trapped bacteria from the airways (25). Nearly half of patients displayed decreased complement 3 (C3) levels at the time of diagnosis in the present study, and with drug use after diagnosis, the abnormal indexes returned to normal. The complement system has a determinant role in defense against infections, and main targets of complement activation are pyogenic organisms and neisserial microbes (26), so the reduction in C3 is probably also an important index of children with PBB. Further research is required to explore the mechanisms by which C3 respond to PBB. Understanding these mechanisms is crucial for developing targeted therapies to manage this chronic respiratory condition.
In our study, the results of sputum culture and sensitivity (C/S) were lower than those of BAL. The British Thoracic Society (BTS) guidelines suggest that prior to diagnosing PBB, it is essential to culture sputum and rule out other potential underlying conditions (10). Nevertheless, most infants diagnosed with PBB were unable to produce sputum, even after attempts at sputum induction in this study. Consequently, acquiring accurate lower respiratory secretions necessitates the use of bronchoscopy, which may be important to clarify pathogens. For the pathogen analysis, the most common pathogens in our study were Moraxella bacteria (12/37, 32.43%) and Streptococcus pneumoniae (11/37, 29.73%). It is the point that distinguishes from the older children. As in previous reports, the bacteria found most in PBB are Hemophilus influenzae, followed by Streptococcus pneumoniae and Moraxella catarrhalis (5,9,12). There is little difference between infants and children in etiology, but this point is important to guide clinicians in the selection of drug therapies.
In the case of PBB, chest CT scan should be conducted after a treatment failure to assess for the potential existence of underlying bronchiectasis (12). However, CT scan is vital for the infants with recurrent wheezing to authenticate bronchopulmonary malformation, especially for infants who show no response or low response to inhale corticosteroid (24). In this study, bronchopulmonary malformation was not identified in any of the infants as determined by CT examination, bronchiectasis was not identified similarly. Our study revealed that uneven lung inflation was the most common imaging changes of pulmonary, but at present there was only limited literature available to the performance of chest CT on infants with PBB. Uneven lung inflation revealed uneven pulmonary perfusion and air trapping in some lung segments of patients, it could be bacterial infections causing some of the airways to swell and narrow. Although the diagnosis of PBB can be attributed mostly to clinical manifestations, it may also be alert by imaging changes, in this case, our imaging findings provide further evidence to PBB for infants with atypical clinical manifestations.
There are several limitations to our study. Firstly, the study was limited to cases of a tertiary center in China. Secondly, the immunological evaluation was not administered in BAL specimens to investigate the local immunological status. Therefore, a larger prospective study is needed to evaluate the clinical characteristics of PBB in infants. Even then, our study provides some new clues for early detection of PBB in infants. In the clinical management of infants presenting with recurrent cough and wheezing, the efficacy of inhaled hormones and bronchodilators is suboptimal. It is essential to consider the possibility of PBB. Additionally, chest CT findings may reveal uneven ventilation as a characteristic manifestation, which may alert clinicians to consider bronchoscopy for a definitive diagnosis combined with medical history.
Conclusions
For infants, wheezing is an important and common clinical indicator when they suffer from PBB. The most common pathogens are Moraxella bacteria and Streptococcus pneumoniae. In particular, the most prominent CT performance of PBB is uneven inflation in infants.
Acknowledgments
We are very appreciative of the children and their families.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-509/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-509/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-509/prf
Funding: This research was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-509/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center of Guangzhou Medical University (No. 2021278B00), and informed consent was obtained from the patients’ parents or legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gilchrist FJ, Ali I, Brodlie M, et al. Developing a core outcome set for children with protracted bacterial bronchitis. ERJ Open Res 2020;6:00344-2019. [Crossref] [PubMed]
- Zhang J, Wurzel DF, Perret JL, et al. Chronic Bronchitis in Children and Adults: Definitions, Pathophysiology, Prevalence, Risk Factors, and Consequences. J Clin Med 2024;13:2413. [Crossref] [PubMed]
- Marchant JM, Masters IB, Taylor SM, et al. Evaluation and outcome of young children with chronic cough. Chest 2006;129:1132-41. [Crossref] [PubMed]
- Narang R, Bakewell K, Peach J, et al. Bacterial distribution in the lungs of children with protracted bacterial bronchitis. PLoS One 2014;9:e108523. [Crossref] [PubMed]
- Chen N, Zhang H, Feng Y. Clinical features and pathogen distributions of microbiological-based protracted bacterial bronchitis in children of different ages in Northeast China. Front Pediatr 2023;11:1163014. [Crossref] [PubMed]
- Marchant J, Masters IB, Champion A, et al. Randomised controlled trial of amoxycillin clavulanate in children with chronic wet cough. Thorax 2012;67:689-93. [Crossref] [PubMed]
- Kantar A. Update on Pediatric Cough. Lung 2016;194:9-14. [Crossref] [PubMed]
- Chang AB, Robertson CF, Van Asperen PP, et al. A multicenter study on chronic cough in children: burden and etiologies based on a standardized management pathway. Chest 2012;142:943-50. [Crossref] [PubMed]
- Øymar K, Mikalsen IB, Crowley S. Protracted bacterial bronchitis in children. Tidsskr Nor Laegeforen 2017;
- Shields MD, Bush A, Everard ML, et al. BTS guidelines: Recommendations for the assessment and management of cough in children. Thorax 2008;63:iii1-iii15. [Crossref] [PubMed]
- Zhang R, Wang L, Gong C, et al. Associated Risk Factors and Diagnostic Value of Fiberoptic Bronchoscopy for Protracted Bacterial Bronchitis in Children. Int J Clin Pract 2023;2023:8116651. [Crossref] [PubMed]
- Gallucci M, Pedretti M, Giannetti A, et al. When the Cough Does Not Improve: A Review on Protracted Bacterial Bronchitis in Children. Front Pediatr 2020;8:433. [Crossref] [PubMed]
- Gilchrist FJ, Aspey M, Bowler R, et al. Protocol for CLASSIC PBB: comparison of lower airway sampling strategies in children with protracted bacterial bronchitis. BMJ Paediatr Open 2022;6:e001722. [Crossref] [PubMed]
- Chen Y, Liu F, Wang C, et al. Molecular Identification and Epidemiological Features of Human Adenoviruses Associated with Acute Respiratory Infections in Hospitalized Children in Southern China, 2012-2013. PLoS One 2016;11:e0155412. [Crossref] [PubMed]
- Kantar A, Chang AB, Shields MD, et al. ERS statement on protracted bacterial bronchitis in children. Eur Respir J 2017;50:1602139. [Crossref] [PubMed]
- Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest 2006;129:260S-83S. [Crossref] [PubMed]
- Asilsoy S, Bayram E, Agin H, et al. Evaluation of chronic cough in children. Chest 2008;134:1122-8. [Crossref] [PubMed]
- Laird P, Totterdell J, Walker R, et al. Prevalence of chronic wet cough and protracted bacterial bronchitis in Aboriginal children. ERJ Open Res 2019;5:00248-2019. [Crossref] [PubMed]
- Lau GTY, Laird P, Stevenson PG, et al. Frequency of protracted bacterial bronchitis and management pre-respiratory referral. J Paediatr Child Health 2022;58:97-103. [Crossref] [PubMed]
- Chang AB, Upham JW, Masters IB, et al. Protracted bacterial bronchitis: The last decade and the road ahead. Pediatr Pulmonol 2016;51:225-42. [Crossref] [PubMed]
- Wurzel DF, Marchant JM, Yerkovich ST, et al. Prospective characterization of protracted bacterial bronchitis in children. Chest 2014;145:1271-8. [Crossref] [PubMed]
- Wiltingh H, Marchant JM, Goyal V. Cough in Protracted Bacterial Bronchitis and Bronchiectasis. J Clin Med 2024;13:3305. [Crossref] [PubMed]
- Zhang XB, Wu X, Nong GM. Update on protracted bacterial bronchitis in children. Ital J Pediatr 2020;46:38. [Crossref] [PubMed]
- Saglani S, Nicholson AG, Scallan M, et al. Investigation of young children with severe recurrent wheeze: any clinical benefit? Eur Respir J 2006;27:29-35. [Crossref] [PubMed]
- Das S, Sockrider M. Protracted Bacterial Bronchitis (PBB) in Children. Am J Respir Crit Care Med 2018;198:11-2. [Crossref] [PubMed]
- Conigliaro P, Triggianese P, Ballanti E, et al. Complement, infection, and autoimmunity. Curr Opin Rheumatol 2019;31:532-41. [Crossref] [PubMed]



