
MtDNA 3243 A>G mutation and recurrent cholangitis after Kasai procedure in biliary atresia: a case report
Highlight box
Key findings
• The mitochondrial DNA (mtDNA) 3243 A>G mutation was identified in a biliary atresia (BA) patient with recurrent cholangitis post-Kasai procedure.
What is known and what is new?
• Post-Kasai cholangitis (30–70% incidence) worsens BA prognosis. MtDNA 3243 A>G cause multisystem disorders, including liver pathologies. Mitochondrial defects promote inflammation/fibrosis in liver diseases.
• Novel association of mtDNA 3243 A>G with recurrent cholangitis in BA. Proposes mitochondrial dysfunction as a potential trigger for post-surgical inflammation. Advocates genetic screening in refractory cholangitis cases.
What is the implication, and what should change now?
• Mitochondrial mutations should be considered in BA patients with unexplained recurrent cholangitis, as they may accelerate disease progression. Comprehensive genetic analysis may help identify high-risk patients and guide therapies. Future studies should validate the role of mtDNA 3243 A>G through functional and cohort analyses.
Introduction
Biliary atresia (BA) is characterized by obliterative cholangiopathy of unknown origin and progressive fibrosclerosing bile ducts to severe cholestasis, liver fibrosis, and cirrhosis (1). Despite improvements in Kasai hepatoportoenterostomy and other perioperative management strategies, many BA patients still require liver transplantation for long-term survival. With an incidence of 30% to 70%, cholangitis is the most frequent complication in patients who have undergone Kasai portoenterostomy. It usually manifests within the first year, particularly in the first 6 months after the procedure. Cholangitis has been shown in multiple studies to be a risk factor for poor outcomes in post-Kasai BA patients (2,3).
Liver tissues are the primary site of several vital metabolic processes, including gluconeogenesis, triacylglyceride oxidation, fatty acid breakdown, amino acid deamination and transamination, and the majority of plasma protein synthesis (4). Hepatocytes are very abundant in mitochondria; on average, each cell has 1,000 mitochondria, which makes up 18% of the total cell volume. Numerous essential cellular processes may be impacted by mitochondrial mutations that seriously harm hepatocytes (5,6). Recent research shows that mitochondrial damage, along with the release of mitochondrial DNA (mtDNA), could trigger the activation of inflammasomes and systemic inflammation in nonalcoholic fatty liver disease and liver failure (7,8). Numerous clinical symptoms, including mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes, have been demonstrated to result from an adenine to guanine substitution at nucleotide 3243 of the mtDNA (m.3243 A>G), which impacts the tRNALeu (UUR) gene. This mutation causes amino acid misincorporation, which could potentially impact inflammation pathways, by altering the processing and steady-state levels of tRNA (9,10). We herein describe a case of the mtDNA 3243A>G mutation in a patient with recurrent cholangitis following a Kasai procedure for BA. We present this case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-592/rc).
Case presentation
The patient in this case was a 7-month-old female infant. When she was 2 months old, her mother observed symptoms of jaundice and stool discoloration, prompting a visit to a local clinic. Subsequently, comprehensive liver function tests were performed at the initial hospital visit. The results revealed elevated levels of total bilirubin (TBIL) at 114.21 µmol/L and direct bilirubin (DBIL) at 60.92 µmol/L. Concurrent urine analysis indicated a 2+ bilirubin presence. Hepatobiliary dynamic imaging was performed. The liver was visualized within 10 minutes post-injection, demonstrating a uniform distribution of the radiotracer. Both kidneys and the bladder were also visualized, with the bladder showing progressive enhancement over time. However, after 24 hours, the absence of radiotracer in the intestinal tract raised concerns for a diagnosis of BA, as no intestinal distribution was observed. To seek further treatment, she was referred to our hospital.
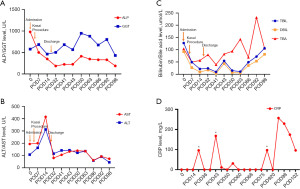
Although the patient was in good condition, she exhibited noticeable scleral yellowing and a brownish hue to her skin. Her stools were creamy grayish-white in appearance. Upon admission, blood test results revealed elevated gamma-glutamyl transpeptidase levels of 578.8 U/L, as well as TBIL at 127.47 µmol/L and DBIL at 92.35 µmol/L (Figure 1). An abdominal ultrasound examination revealed an unclear display of the common bile duct. The abdominal ultrasound performed with the patient fasting revealed a gallbladder measuring 1.5 cm × 0.3 cm × 0.4 cm, with a wall thickness of 0.25 cm. The gallbladder exhibited a rigid shape and the inner wall appeared irregular. A follow-up ultrasound approximately 2 hours after breastfeeding demonstrated no changes in the gallbladder’s size or morphology, suggesting a diagnosis of BA. Ultrasound elastography was performed, with multiple random points selected on the right lobe of the liver to measure tissue elasticity. The average elasticity value obtained was 12.1 kilopascals (kPa).
The patient underwent laparoscopic exploration surgery. Under direct visualization with the laparoscope, the liver appeared enlarged and brownish-brown, with nodular changes and thickened surface capillaries resembling spider angiomas. The gallbladder, which was small and underdeveloped, had a cord-like appearance and was located within the liver fissure. Due to the enlarged liver, the cholangiography could not be completed during the laparoscopic procedure. Consequently, an open surgery, specifically a Kasai procedure, was performed at the request of the parents. Samples of liver tissue, the excised gallbladder, and the fibrous block at the hepatic hilum were collected and sent for pathological examination.
The pathology report revealed significant findings as follows—liver: the histological features align with early biliary fibrosis, attributed to extrahepatic biliary obstruction, and classified as Ishak grade 4. Immunohistochemical analysis showed cytomegalovirus (CMV) negativity and cytokeratin 7 (CK7) positivity in bile duct epithelium. Special staining results indicated positivity for Periodic Acid-Schiff (PAS) and Masson stains, negativity for copper, and positivity for mesh fiber. Gallbladder: the gallbladder was underdeveloped. Fibrous block at the hepatic hilum: the specimen comprised small fibrous tissues characterized by scattered, small lumens lined by flat epithelium. Lymphocytic infiltration surrounding the tubular structures suggested an inflammatory response. Additionally, proliferation and hypertrophy of the nerve plexus were observed, accompanied by blood vessels of varying wall thicknesses in the peritubular region.
Post-surgery, the patient received prophylactic antibiotics to prevent cholangitis. Intravenous meropenem (20 mg/kg per dose, every 8 hours) was administered for two weeks, followed by alternating oral cefdinir dispersible tablets and co-trimoxazole tablets every two weeks. The patient experienced darker stool color, lighter urine color, and a gradual reduction in skin and scleral jaundice. Nineteen days after the operation, the patient was discharged with follow-up blood biochemistry tests revealing an r-glutamyl transferase level of 463.7 U/L, TBIL at 21.5 µmol/L, and DBIL at 9.05 µmol/L (Figure 1). Despite initial improvement, the patient suffered recurrent episodes of cholangitis within the following 3 months, leading to three hospital readmissions. During these admissions, comprehensive diagnostic evaluations were performed: serial C-reactive protein (CRP) levels were monitored (Figure 1D), revealing persistent inflammation with a peak value of 257 mg/L; peripheral blood cultures were collected during febrile episodes (body temperature ≥38.5 ℃), all of which showed no bacterial growth after 120 hours of incubation. Intravenous meropenem (20 mg/kg every 8 hours) was initiated empirically during each admission. Following the first two episodes, fever resolved within 72 hours, and the patient was discharged afebrile. However, during the final admission, persistent fever (>39 ℃) and rising CRP levels prompted escalation to fluconazole (for fungal coverage) and intravenous immunoglobulin (IVIG). Despite these interventions, no clinical improvement was observed. Linezolid was subsequently added to target suspected drug-resistant pathogens, yet inflammatory markers remained elevated. Furthermore, liver elasticity measurements, assessed by ultrasound elastography, showed a continuous increase, reaching 42.0 kPa, indicative of worsening liver fibrosis. Magnetic resonance cholangiopancreatography conducted on the 77th postoperative day revealed dilated intrahepatic bile ducts (Figure 2).

Given the patient’s complex clinical course, a genetic investigation was conducted using whole-exome sequencing (WES) after informed consent. This revealed a pathogenic variant in mtDNA at position 3243 (A>G) with a frequency of 9,195/(9,195+2,432) (79.08%). Family pedigree analysis showed that the patient’s mother had a mutation frequency of 3,931/(3,931+21,076) (15.72%) at the same locus (Figure 3). Due to the progression of liver disease and the inability to control cholangitis, the patient underwent liver transplantation at an external hospital 5 months after the initial surgery. The patient recovered successfully and remained stable at the last follow-up at 20 months of age.

All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Cholangitis remains the predominant complication following the Kasai procedure for BA, with numerous studies underscoring its pivotal role in influencing the long-term prognosis of affected patients. The recurrent bouts of cholangitis contribute to the progression of liver fibrosis, ultimately leading to it becoming a critical indication for liver transplantation. Despite the significant impact of postoperative cholangitis, the underlying causes of these episodes remain not fully elucidated.
Here, we describe the first instance of mtDNA 3243 A>G mutation in a patient with BA with recurrent cholangitis. The mtDNA 3243 A>G mutation occurs at the conserved nucleotide in the dihydrouridine loop of the tRNALeu (UUR) and is most frequently associated with mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) (9). The phenotypic expression of the mtDNA 3243 A>G mutation can be highly variable and has been detected in many diseases, such as type 2 diabetes and deafness syndrome, progressive external ophthalmoplegia, VACTERL syndrome, membranous nephropathy, colon cancer, acute pancreatitis, and hepatic failure (11-13). Of all these mutations and polymorphisms, only a few can be linked to a known phenotypic effect. In a 6-year prospective study of 151 patients with the mtDNA 3243 A>G mutation, it was found that their phenotypes were highly variable. Among these patients, 57 cases could not be linked to any known phenotypes and were classified into an “other” group (14). Among children diagnosed with multiple respiratory chain enzyme deficiencies, mtDNA depletion has been observed in 50% (50 out of 100 cases), with a significant proportion of these patients—64% (32 out of 50)—experiencing severe liver involvement from neonatal onset. Notably, the underlying mutations remain unidentified in half of these cases, highlighting the existence of additional genetic heterogeneity (15). In a separate study, whole exome sequencing was conducted on three pediatric patients with acute liver failure, revealing pathogenic mutations in MPV17, SERAC1, and NOTCH2 genes, despite the absence of typical clinical manifestations associated with these genetic alterations (16). Based on previous findings, it is plausible that rare genetic factors contribute to acute liver failure in some patients, and further exploration through next-generation sequencing may uncover novel mitochondrial phenotypes. The presence of this mutation in our patient may suggest a potential contribution to the pathogenesis of recurrent cholangitis following the Kasai procedure.
The inflammatory and fibrogenic processes in BA involve bile duct injury and cholangiocyte apoptosis, leading to liver fibrosis, cirrhosis, and potentially end-stage liver disease (17). Deregulated inflammatory responses are crucial in BA, driving disease progression and impairing outcomes. Mitochondrial dysfunction is a key factor in various liver diseases, including alcoholic liver disease (ALD), non-alcoholic fatty liver disease (NAFLD), liver fibrosis, and drug-induced liver injury (18). However, the specific mechanisms underlying hepatic mitochondrial dysfunction in BA remain unclear. Mitochondrial dysfunction in BA may arise from the accumulation of toxic bile acids, which induce oxidative stress, exacerbate inflammation, and lead to cell damage, including cell senescence and mitochondrial dysfunction. Mitochondria play a crucial role in adenosine triphosphate (ATP) and reactive oxygen species (ROS) production, signaling, cytosolic Ca2+ pumping, and apoptosis regulation. Mutations in mtDNA can disrupt cellular energy transduction, impair liver energy metabolism, and affect liver regeneration and inflammatory response capabilities (6,19). Koh et al. discovered 34 prevalent non-synonymous mtDNA protein-coding gene variants in patients with BA. A comprehensive 3D structural examination uncovered mutations within pivotal areas of respiratory chain complexes I through V, which play roles in subunit assembly, proton pumping, and supercomplex assembly. Notably, the intensity of chronic liver damage and liver malfunction in BA patients was strongly linked to the degree of hepatic failure, hinting that mtDNA mutations could intensify hepatopathy and serve as foundational mechanisms underpinning BA-related pathology (6). Nakajima et al. found that cell senescence and mtDNA damage progression in BA patients undergoing Kasai portoenterostomy and liver transplantation depended on the clinical stage and could serve as liver transplantation indicators (19). Lane et al. observed mitochondrial dysfunction in severe liver failure cases unrelated to primary mitochondrial disorders. Among 45 patients investigated for liver transplantation due to various reasons, 9 had BA, and 20–50% showed variable mitochondrial alterations (20). However, the role of these alterations in BA pathomechanism remains unclear, possibly linked to specific stages of hepatocellular dysfunction, supported by low mtDNA copy numbers in leukocytes of BA patients, indicating inflammation and secondary mtDNA damage (21). Moreover, Decreased mtDNA copy number was also associated with increased risks of BA, severe fibrosis, jaundice, and hepatic dysfunction (22). Notably, the mtDNA 3243 A>G mutation, associated with various mitochondrial disorders and liver pathologies, was detected in our patient. This suggested a potential link between mitochondrial dysfunction and recurrent cholangitis following the Kasai procedure, although the exact mechanism contributing to the patient’s phenotype remains unknown. Future studies should validate the functional impact of the mtDNA 3243 A>G mutation through experimental models and large-scale cohorts to define its clinical relevance. In addition, although no clinically significant pathogenic single nucleotide variants (SNVs) or large-fragment copy number variants (CNVs) were identified in the nuclear genes of this patient based on the WES results, numerous variants of uncertain significance (VUS) were detected. These VUS may require further validation in future studies to better understand their potential roles in the disease.
Conclusions
In conclusion, this case report highlights a rare association between BA, recurrent cholangitis, and the mtDNA 3243 A>G mutation. Our findings suggest that mitochondrial dysfunction, driven by this mutation, may contribute to recurrent cholangitis post-Kasai procedure. This underscores the importance of genetic evaluation, particularly for mitochondrial mutations, in BA patients with recurrent cholangitis. Comprehensive genetic analysis could offer insights into disease progression and guide personalized therapies.
Acknowledgments
None
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-592/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-592/prf
Funding: This study was supported by a grant from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-592/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’s parents for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qiao G, Li L, Cheng W, et al. Conditional probability of survival in patients with biliary atresia after Kasai portoenterostomy: a Chinese population-based study. J Pediatr Surg 2015;50:1310-5. [Crossref] [PubMed]
- Gunadi, Gunawan TA, Widiyanto G, et al. Liver transplant score for prediction of biliary atresia patients' survival following Kasai procedure. BMC Res Notes 2018;11:381.
- Jiang H, Gao P, Chen H, et al. The Prognostic Value of CD8(+) and CD45RO(+) T Cells Infiltration and Beclin1 Expression Levels for Early Postoperative Cholangitis of Biliary Atresia Patients after Kasai Operation. J Korean Med Sci 2018;33:e198. [Crossref] [PubMed]
- Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev 2010;90:1165-94. [Crossref] [PubMed]
- Lang C, Berardi S, Schäfer M, et al. Impaired ketogenesis is a major mechanism for disturbed hepatic fatty acid metabolism in rats with long-term cholestasis and after relief of biliary obstruction. J Hepatol 2002;37:564-71. [Crossref] [PubMed]
- Koh H, Park GS, Shin SM, et al. Mitochondrial Mutations in Cholestatic Liver Disease with Biliary Atresia. Sci Rep 2018;8:905. [Crossref] [PubMed]
- Marques PE, Amaral SS, Pires DA, et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 2012;56:1971-82. [Crossref] [PubMed]
- Xu L, Zhou J, Che J, et al. Mitochondrial DNA enables AIM2 inflammasome activation and hepatocyte pyroptosis in nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol 2021;320:G1034-44. [Crossref] [PubMed]
- Ahmed ST, Craven L, Russell OM, et al. Diagnosis and Treatment of Mitochondrial Myopathies. Neurotherapeutics 2018;15:943-53. [Crossref] [PubMed]
- Tranah GJ, Katzman SM, Lauterjung K, et al. Mitochondrial DNA m.3243A > G heteroplasmy affects multiple aging phenotypes and risk of mortality. Sci Rep 2018;8:11887. [Crossref] [PubMed]
- Lorenc A, Bryk J, Golik P, et al. Homoplasmic MELAS A3243G mtDNA mutation in a colon cancer sample. Mitochondrion 2003;3:119-24. [Crossref] [PubMed]
- Tsujita Y, Kunitomo T, Fujii M, et al. A surviving case of mitochondrial cardiomyopathy diagnosed from the symptoms of multiple organ dysfunction syndrome. Int J Cardiol 2008;128:e43-5. [Crossref] [PubMed]
- Kishnani PS, Van Hove JL, Shoffner JS, et al. Acute pancreatitis in an infant with lactic acidosis and a mutation at nucleotide 3243 in the mitochondrial DNA tRNALeu(UUR) gene. Eur J Pediatr 1996;155:898-903. [Crossref] [PubMed]
- de Laat P, Rodenburg RR, Roeleveld N, et al. Six-year prospective follow-up study in 151 carriers of the mitochondrial DNA 3243 A>G variant. J Med Genet 2021;58:48-55. [Crossref] [PubMed]
- Sarzi E, Bourdon A, Chrétien D, et al. Mitochondrial DNA depletion is a prevalent cause of multiple respiratory chain deficiency in childhood. J Pediatr 2007;150:531-4, 534.e1-6.
- Vilarinho S, Choi M, Jain D, et al. Individual exome analysis in diagnosis and management of paediatric liver failure of indeterminate aetiology. J Hepatol 2014;61:1056-63. [Crossref] [PubMed]
- Chusilp S, Lee C, Li B, et al. A novel model of injured liver ductal organoids to investigate cholangiocyte apoptosis with relevance to biliary atresia. Pediatr Surg Int 2020;36:1471-9. [Crossref] [PubMed]
- Marchi S, Guilbaud E, Tait SWG, et al. Mitochondrial control of inflammation. Nat Rev Immunol 2023;23:159-73. [Crossref] [PubMed]
- Nakajima Y, Yamazaki Y, Gao X, et al. Association between mitochondrial and nuclear DNA damages and cellular senescence in the patients with biliary atresia undergoing Kasai portoenterostomy and liver transplantation. Med Mol Morphol 2022;55:131-45. [Crossref] [PubMed]
- Lane M, Boczonadi V, Bachtari S, et al. Mitochondrial dysfunction in liver failure requiring transplantation. J Inherit Metab Dis 2016;39:427-36. [Crossref] [PubMed]
- Tiao MM, Lin TK, Kuo FY, et al. Early stage of biliary atresia is associated with significant changes in 8-hydroxydeoxyguanosine and mitochondrial copy number. J Pediatr Gastroenterol Nutr 2007;45:329-34. [Crossref] [PubMed]
- Udomsinprasert W, Poovorawan Y, Chongsrisawat V, et al. Leukocyte mitochondrial DNA copy number as a potential biomarker indicating poor outcome in biliary atresia and its association with oxidative DNA damage and telomere length. Mitochondrion 2019;47:1-9. [Crossref] [PubMed]


