
Clinical characteristics of single human rhinovirus infection and co-infection in the respiratory tract of children
Highlight box
Key findings
• Human rhinovirus (HRV) is a leading pathogen in childhood respiratory infections, often co-infecting with adenovirus (ADV) and respiratory syncytial virus (RSV).
• HRV-ADV co-infection is strongly linked to fever, while HRV-RSV co-infection correlates with higher rates of cough and pneumonia.
• HRV co-infections accounted for 84.62% of mixed respiratory infections.
What is known and what is new?
• HRV causes respiratory infections in children, but its role in mixed infections and clinical outcomes is poorly understood.
• This study highlights HRV’s high co-infection rates with ADV and RSV and its distinct clinical manifestations, such as fever and respiratory complications.
What is the implication, and what should change now?
• Clinicians should consider HRV co-infections in children with fever or pneumonia for accurate diagnosis and management.
• Future studies should explore targeted interventions for HRV co-infections and their underlying mechanisms.
Introduction
Respiratory tract infections are common. The Global Burden of Disease predicted that lower respiratory tract infections were the fifth leading cause of death around the world in 2015 (1). Approximately 2.5 million people die yearly from such infections worldwide, including children and those who are vulnerable due to compromised immunity (2-4). Between 2012 and 2021, out of 15,645 patients with acute respiratory infections (ARI), 1,180 (7.5%) were diagnosed with respiratory viral (RV) infections, and 820 (69.5%) of these cases were in children under 5 years old (5). While rapid diagnostic tests are available for several important pathogens implicated in acute respiratory illness, and respiratory pathogen panels are routinely used in some settings, the identification of pathogens remains challenging in resource-limited environments where diagnostics are not readily available (6,7). In such settings, the complexity of clinical manifestations and the presence of multiple potential pathogens can hinder the rapid and accurate identification of the causative agents. A previous study has shown that in some local clinics, there may be a tendency to treat patients according to their relevant experience, resulting in indiscriminate antibiotic use and increased drug resistance (8). At present, there is a growing focus in clinics on respiratory infections related to influenza virus, respiratory syncytial virus (RSV), and adenovirus (ADV)-related respiratory infections (9-12), as well as other important pathogens such as SARS-CoV-2, common cold coronaviruses (ccCoV), human metapneumovirus (HMPV), and parainfluenza viruses (PIV). These pathogens, along with human rhinovirus (HRV) (13,14), play a significant role in acute respiratory illnesses, particularly in vulnerable populations such as young children and the elderly.
HRVs are the leading cause of colds and asthma exacerbation, otitis media, angina, and acute bronchiolitis, which are common acute infections of the respiratory tract in infants and young children (15). Research indicated that HRV was detected in the respiratory tract of 80–85% of children with acute asthma (16). Significantly, studies have shown that a combined infection of HRV and other respiratory viruses, such as RSV, can exacerbate a patient’s condition. For example, co-infection with HRV and RSV has been associated with increased severity of acute bronchiolitis (17). Additionally, HRV has been linked to a higher risk of childhood asthma exacerbations, with HRV-C strains being particularly associated with more severe attacks than other HRV serotypes and other respiratory viruses (18). To gain a more comprehensive understanding of HRV infection, the present study measured the positive rate of HRV in the respiratory tract of children and compared the difference in symptoms between HRV single infection and co-infection with other viruses. The results may support promoting the diagnosis and treatment of respiratory tract infections caused by HRV. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-24-79/rc).
Methods
Data
This prospective study involving 438 inpatients was conducted at The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China (Hefei Infectious Disease Hospital), China, from February to April 2021. Information related to patient demographics, symptoms, physical examinations, and laboratory results was collected. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China (No. 2024-RE-283). Informed consent was obtained from the guardians of the patients.
The inclusion criteria were as follows: (I) children aged ≤10 years, regardless of gender; (II) consecutive cases of acute upper respiratory tract infection in children; (III) blood routine or C-reactive protein (CRP) examination results suggesting a viral infection; (IV) the diagnostic criteria for respiratory tract infection were met as per Zhu Futang Practical Pediatrics (2011, 7th ed.). The exclusion criteria for this study were as follows: (I) patients who had had pneumonia in the preceding three months; (II) patients with blood routine examination results that suggested a bacterial infection, such as increased neutrophil count and elevated white blood cell count, and those with CRP levels significantly elevated to a degree typically associated with bacterial infections rather than viral infections; (III) patients with a history of premature birth, asthma, severe cardiovascular problems, and liver, kidney, or other diseases; (IV) children with impaired airway clearance ability due to neuromuscular or neurological conditions.
Participants must exhibit symptoms such as fever, cough, and wheezing, with viral pneumonia often presenting with wheezing. Chest pain may be reported by older children, while hemoptysis is rare. Infants less than 2 months of age may not have fever but may show signs like frothy sputum, apnea (cessation of breathing), or choking cough. Additionally, participants should have a history of persistent fever with cough for more than 3 to 5 days. Physical examination should reveal signs such as an increased respiratory rate (RR) and moist rales, which are indicative of pneumonia, particularly in infants and young children. Mycoplasma pneumonia (MP) often presents without rales. The RR should be increased based on the following age-specific criteria when observed calmly for 1 minute: less than 2 months of age with ≥60 breaths per minute; 2 months to 1 year with ≥50 breaths per minute; 1 to 5 years with ≥40 breaths per minute; and over 5 years with ≥30 breaths per minute. As the disease progresses, participants may develop rapid and shallow breathing, chest wall indrawing during inhalation, nasal flaring, the three-muscle sign (triple sign), moaning, and cyanosis. Behavioral changes such as irritability, lethargy, somnolence, and refusal to eat may also be present.
Sample collection
The specimens for the detection of pathogens were collected using nasopharyngeal swabs. After inserting the swab into and clearing the nasal cavity, the swab was rotated several times, removed, and then immersed in a tube containing a 3-mL virus preservation solution (an isotonic salt solution, tissue culture solution, or a phosphate buffer). The samples were stored at 4 °C for use within 48 h or below −70 °C if kept for more than three months.
Polymerase chain reaction (PCR) detection
Viral nucleic acid detection of common respiratory viruses was conducted using a multiplex PCR product provided by Sansure Biotech Inc. (Changsha, China). The pathogens included HRV, RSV, ADV, influenza viruses A and B, respectively, and MP.
Multiplex PCR
Multiplex PCR offers significant advantages in pathogen detection, especially when identifying multiple pathogens. By simultaneously detecting multiple pathogens or pathogen genes, multiplex PCR greatly enhances detection efficiency and shortens the turnaround time. This method is particularly useful for cases with mixed or multiple infections, or when the etiology is unclear, providing more comprehensive and rapid diagnostic information for clinicians. Additionally, multiplex PCR is easy to perform, relatively cost-effective, and suitable for large-scale screening and routine clinical applications (19).
Statistical analysis
The data were processed using the SPSS Statistics 26.0 software program. The means of normally distributed continuous variables were compared by one-way analysis of variance and t-tests. Non-normally distributed continuous data were compared using a Mann-Whitney U test, Kruskal-Wallis test or Chi-squared test, and P<0.05 was considered statistically significant.
Results
The viral etiology of respiratory infections
As shown in Table 1, a total of 438 specimens were collected. There were 256 positive specimens, accounting for 58.45% of the total specimens, and 182 negative specimens without pathogens. The highest positive rate was recorded for HRV (30.36%), followed by RSV (11.39%), and ADV (7.29%). There were only two cases with positive MP results, and no flu virus infection cases were detected. Notably, there were 39 co-infection cases, accounting for 8.90% of the total specimens; HRV and ADV co-infection, RSV, and MP were 4.57%, 2.05%, and 0.91%, respectively, which accounted for 84.62% of the co-infection cases.
Table 1
| Pathogen | Positive number | Positive rate (%) |
|---|---|---|
| Single infection | ||
| HRV | 133 | 30.37 |
| RSV | 50 | 11.42 |
| ADV | 32 | 7.31 |
| MP | 2 | 0.46 |
| Flu A/B | 0 | 0 |
| Co-infection | ||
| HRV ADV | 20 | 4.57 |
| HRV RSV | 9 | 2.05 |
| RSV ADV | 5 | 1.14 |
| HRV MP | 4 | 0.91 |
| ADV RSV | 1 | 0.23 |
ADV, adenovirus; Flu A/B, influenza A and B; HRV, human rhinovirus; MP, Mycoplasma pneumoniae; RSV, respiratory syncytial virus.
Demographic characteristics
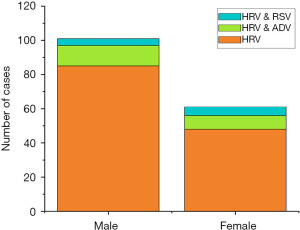
Considering the sample size in the three groups, single HRV infection, HRV and ADV, and HRV and RSV co-infections were analyzed in this study. The demographic-based results are shown in Figures 1,2. There were 101 males (62.3%) and 61 females (37.7%) among the 162 positive HRV cases. The proportion of males and females among the patients with a single HRV infection was 63.9% and 36.1%, respectively. In HRV and ADV co-infection, the proportion of males and females was 60% and 40%, respectively. The results indicated that the proportion of males was statistically significant (P<0.05) for HRV single infection but not in co-infections.
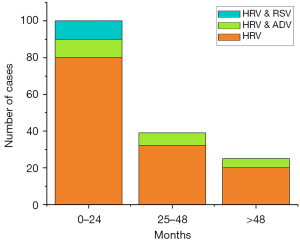
In the age distribution comparison, there were 100 patients in the 0–24-month age group, 39 patients in the 25–48-month age group, and 23 patients in the older than 48-month age group. The proportion of HRV infection related infants aged 0–24-month was higher than in the other age groups. Notably, patients with HRV and RSV co-infection were all in the 0–24-month age group. However, in cases with HRV and ADV co-infection, the distribution ages were uniform (P=0.07).
Laboratory examination results
The blood routine test results were further analyzed. As shown in Table 2, the mean number of white blood cells for HRV, HRV and ADV, and HRV and RSV were 11.26×109/L, 11.11×109/L, and 9.32×109/L, respectively. The median values of white blood cell-lymphocyte percentage in the three infectious conditions were 35.00%, 25.30%, and 41.40%, and the median values of white blood cell-granulocyte percentage were 53.70%, 63.55%, and 48.20%, respectively. Statistical analysis indicated a significant difference in the number of leukocytes, W-SCR and W-LCR results. The P value were 0.01, 0.049 and 0.047 respectively. In the CRP test, the median values of HRV, HRV and ADV, and HRV and RSV were 10.00, 13.48, and 1.29 mg/L, respectively. There was no significant difference in CRP between the three infection types, based on the statistical results (P=0.57).
Table 2
| Laboratory examination | HRV infection (n=133) | HRV & ADV coinfection (n=20) | HRV & RSV coinfection (n=9) | P value |
|---|---|---|---|---|
| WBC, ×109/L | 11.26 (5.39) | 11.11 (9.00) | 9.32 (5.45) | 0.01 |
| Lymphocyte, % | 35.00 (23.55–52.50) | 25.30 (17.85–38.62) | 41.40 (29.95–55.60) | 0.049 |
| Granulocyte, % | 53.70 (36.00–68.65) | 63.55 (51.90–74.93) | 48.20 (29.85–61.30) | 0.047 |
| Peak of CRP, mg/L | 10.00 (1.51–35.82) | 13.48 (8.40–46.58) | 1.29 (0.45–22.84) | 0.57 |
Data are presented as mean (SD) or median (IQR). ADV, adenovirus; CRP, C-reactive protein; HRV, human rhinovirus; IQR, interquartile range; RSV, respiratory syncytial virus; SD, standard deviation; WBC, white blood cell count.
Symptoms and diagnosis
The HRV infection-related symptoms were compared, including fever, cough, rhinorrhea, and shortness of breath (see Table 3). The percentage of cases including fever in the HRV, HRV and ADV, and HRV and RSV groups were 67.7%, 100%, and 44.4%, respectively. The HRV and ADV group had significantly higher (P=0.003) fever values compared with the two other groups. However, the HRV and RSV groups reflected a 100% result for cough symptoms, which differed significantly from the other groups (P=0.04). In contrast, the distribution of rhinorrhea and shortness of breath symptoms in the three groups was uniform, with no observable significant difference.
Table 3
| Category | HRV infection (n=133) | HRV & ADV coinfection (n=20) | HRV & RSV coinfection (n=9) | P value |
|---|---|---|---|---|
| Symptoms | ||||
| Fever | 90 (67.7) | 20 (100.0) | 4 (44.4) | 0.003 |
| Cough | 105 (78.9) | 12 (60.0) | 9 (100.0) | 0.04 |
| Rhinorrhea | 42 (31.6) | 4 (20.0) | 5 (55.6) | 0.17 |
| Shortness of breath | 36 (27.1) | 4 (20.0) | 3 (33.3) | 0.72 |
| Diagnosis | ||||
| Rhinitis | 20 (15.0) | 6 (30.0) | 0 (0.0) | 0.09 |
| Bronchitis | 1 (0.8) | 0 (0.0) | 0 (0.0) | 0.90 |
| Pneumonia | 86 (64.7) | 10 (50.0) | 9 (100.0) | 0.03 |
| Myocardial damage | 14 (10.5) | 4 (20.0) | 0 (0.0) | 0.25 |
Data are presented as n (%). ADV, adenovirus; HRV, human rhinovirus; RSV, respiratory syncytial virus.
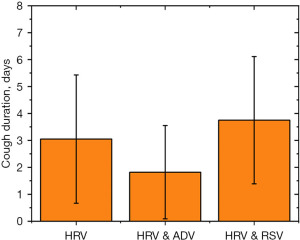
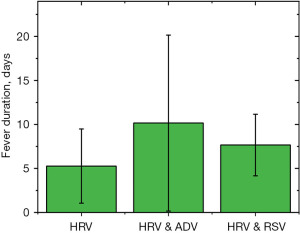
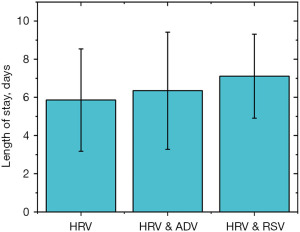
The incidence analysis revealed that the rate of pneumonia was 64.7%, 50%, and 100% in the HRV, HRV and ADV, and HRV and RSV groups, respectively. The total infection rate of pneumonia in the HRV and RSV groups was 100.0%. As shown in Figures 3-5, the time period that fever, coughing, and hospital stay lasted were further compared between the three groups. The average cough duration in the three groups was 3.05, 1.82, and 3.75 days, and the length of hospital stay was 5.86, 6.35, and 7.11 days, respectively, which was not statistically significant (P=0.33). However, the fever time in the three groups was 5.27, 10.16, and 7.66 days, respectively, which was statistically significant (P=0.004). The time of fever and cough in the HRV and ADV group reflected large individual differences.
Discussion
HRV though extensively studied, remains enigmatic in pediatric populations, particularly in coinfections and their impact across different seasons, warranting further investigation into its clinical nuances. Very few studies have been conducted on RVs, and clinicians sometimes neglect the dangers of HRV infections in China. This study calculated the positive rate and explored the clinical characteristics of HRV infection. Overall, 438 samples containing respiratory pathogens were analyzed, and the positivity rate was 58.45%. The most common virus was HRV (30.36%), followed by RSV (11.39%) and ADV (7.29%). There were 39 co-infection cases, accounting for 8.90% of the total specimens. An HRV and ADV co-infection or RSV accounted for 74.36% of the co-infection cases.
“Co-infection” refers to the situation where two or more pathogens are infecting the same host at the same time. Co-infection may be related to prolonged periods of viral persistence in the mucosa of the respiratory tract. The effects of gender and age on the results of HRV infection were explored in this study; the results showed that more males than females had been affected in the HRV single-infection condition; these results were the same as in other studies (20,21). In addition, younger patients, particularly those who were <2 years, reflected higher positive cases. Significantly, HRV and ADV co-infection cases were all found in patients under 2 years old, indicating that infants and toddlers were more susceptible to HRV compared with older children. Pediatric patients have an incompletely established immune system and are immunocompromised, so they are susceptible to infection by pathogens (22).
In blood routine analysis, leukocyte, neutrophil, and lymphocyte levels were significant when comparing a single RV infection with co-infection (P<0.05). The number of leukocytes in a single condition was higher than in mixed infection cases. However, comparatively, HRV and RSV co-infection showed the highest neutrophil level, and HRV and ADV co-infection had the highest lymphocyte level. Although some studies showed that the neutrophil percentage in viral co-infection was higher than that in a single infection (23,24), the level of leukocytes in children is significantly different from that in adults. Lymphocyte and neutrophil levels change according to the age of children. Neutrophils increase gradually after the age of 2 years, and the 4–6 years old children have a similar lymphocyte and neutrophil levels. After the age of 6 years, neutrophils continue to increase and lymphocytes decrease (25,26). Therefore, using blood routine or CRP diagnosis results can be challenging in terms of establishing whether a single or co-infection is involved in children, as well as the infection severity. From the complete blood counts (CBC) and CRP data, we observed signs that may suggest an active immune response, such as changes in white blood cell counts, which led us to speculate that there may be alterations in T cell function and other potential immunological effects. However, it is important to note that CBCs and CRP data alone are not sufficient to comprehensively and accurately assess T cell function and other immunological effects; these speculations require further immunological testing for validation. The preliminary findings of our study suggest that future research could consider incorporating more comprehensive immunological tests, such as flow cytometry for T cell subset analysis, to delve deeper into these potential immunological mechanisms.
Symptom analysis revealed significant differences between fever and cough characteristics in the three groups (P<0.05). Compared with HRV single infection (78.9%), the incidence of the cough symptoms in group co-infected with HRV and RSV (100%) was substantially higher. This is consistent with the higher incidence rate of cough in cases of RSV (27). The reason for this is that the epithelial cells in the trachea, bronchioles, and alveoli are the main target cells of an RSV infection (28), which causes the shedding of airway cilia and epithelial cells. These neutrophils, cell debris, and lymphocytes accumulate in the airway, resulting in airway obstruction. Concurrently, the excessive secretion of mucus and airway edema will aggravate airway obstruction (29). In the group of patients co-infected with HRV and ADV, fever was observed in all patients (100%), which aligns with the common presentation of high fever above 39 °C at the onset of RSV infection (30). Fever symptoms are related to the release of inflammatory mediators and autoimmune dysfunction. Studies have shown that interleukin (IL)-4, IL-6, and tumor necrosis factor increased in children with severe ADV pneumonia. The inhibition of T-cell functioning may directly result from ADV or non-specific inhibition caused by an impaired immune function in severe cases of infection (31). It is noted that the infection rate of HRV with RSV pneumonia was much higher than in the other groups, which indicated that HRV and RSV may aggravate respiratory tract infections.
There are some limitations to this research. First, the sample size is relatively small, and only several common viruses were tested among the recruited patients; as such, co-infection with bacteria requires further investigation. Second, children were examined at a single location, thereby limiting the validity of virus-specific clinical correlations in different contexts. Third, similar studies should be conducted to include long-term follow-up time frames to collect additional information. Lastly, the children were enrolled from February to April. Therefore, it cannot effectively represent the situation of the whole year or the situation of each season. To enhance the reproducibility and interpretability of our study, we need to further optimize our research design. For instance, we will consider expanding the sample size and conducting multicenter studies.
Although our study focused on symptomatic patients, it is important to note that asymptomatic patients can also play a significant role in the transmission and clinical presentation of respiratory infections. For example, a study has shown that asymptomatic patients can have similar viral loads to symptomatic patients but may clear the virus more quickly and have more normal clinical indicators (32). This suggests that asymptomatic patients may contribute to the spread of infection but may have different clinical outcomes compared to symptomatic patients. Future studies should consider including asymptomatic patients to provide a more comprehensive understanding of the epidemiology and clinical impact of respiratory infections.
Conclusions
The statistics of HRV causing respiratory tract infections, based on age, gender, laboratory examination, symptoms, and the process of recovery, are anticipated to contribute to the diagnosis of clinical respiratory tract infection. Combining the optimization of pathogen detection technology and multiplex PCR may be able to reduce the misdiagnosis of infectious diseases, promote appropriate drug treatment, and provide adequate evidence for clinical decision-making in the future.
Acknowledgments
We are particularly grateful to all the people who have given us help with our article.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-79/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-79/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-79/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-79/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China (No. 2024-RE-283). Informed consent was obtained from the guardians of the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017;17:1133-61. [Crossref] [PubMed]
- Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022;9:137-50. [Crossref] [PubMed]
- Perin J, Mulick A, Yeung D, et al. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health 2022;6:106-15. Erratum in: Lancet Child Adolesc Health 2022;6:e4. [Crossref] [PubMed]
- Cannesson A, Elenga N. Community-Acquired Pneumonia Requiring Hospitalization among French Guianese Children. Int J Pediatr 2021;2021:4358818. [Crossref] [PubMed]
- Cui A, Xia B, Jiang H, et al. Prevalence and genetic diversity of human rhinovirus among patients with acute respiratory infections in China, 2012-2021. J Med Virol 2024;96:e29582. [Crossref] [PubMed]
- El FS, Seffar M, Kettani C, et al. Procalcitonin values in respiratory infections children under five years old viral infections versus bacterial infections. J Respir Dis Med 2019. doi:
10.15761/JRDM.1000101 . - Stower H. Rapid lower respiratory tract infectious diagnosis. Nat Med 2019;25:1189. [Crossref] [PubMed]
- Takei H, Takeuchi N, Hoshino T, et al. Bacteriological analysis of Neisseria lactamica isolated from the respiratory tract in Japanese children. J Infect Chemother 2021;27:65-9. [Crossref] [PubMed]
- Nishioka K, Kyo M, Nakaya T, et al. Proteins produced by Streptococcus species in the lower respiratory tract can modify antiviral responses against influenza virus in respiratory epithelial cells. Microbes Infect 2021;23:104764. [Crossref] [PubMed]
- Yao LH, Wang C, Wei TL, et al. Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017-2018. Virol J 2019;16:78. [Crossref] [PubMed]
- Liu P, Xu M, Lu L, et al. Resurgence of common respiratory viruses and mycoplasma pneumoniae after ending the zero-COVID policy in Shanghai. Sci Rep 2025;15:1765. [Crossref] [PubMed]
- Aizawa Y, Ikuse T, Izumita R, et al. Human Rhinovirus as a Cause of Fever in Neonates and Young Infants During the COVID-19 Pandemic, 2020-2022. Pediatr Infect Dis J 2024;43:130-5. [Crossref] [PubMed]
- Zhao Y, Shen J, Wu B, et al. Genotypic Diversity and Epidemiology of Human Rhinovirus Among Children With Severe Acute Respiratory Tract Infection in Shanghai, 2013-2015. Front Microbiol 2018;9:1836. [Crossref] [PubMed]
- Triantafilou M, Ramanjulu J, Booty LM, et al. Human rhinovirus promotes STING trafficking to replication organelles to promote viral replication. Nat Commun 2022;13:1406. [Crossref] [PubMed]
- Sanchez-Codez MI, Moyer K, Benavente-Fernández I, et al. Viral Loads and Disease Severity in Children with Rhinovirus-Associated Illnesses. Viruses 2021;13:295. [Crossref] [PubMed]
- Klaiber N. The Role of Rhinovirus in the Pathogenesis and Acute Exacerbation of Asthma. Clin Pulm Med 2018;25:12-9.
- Ding Q, Xu L, Zhu Y, et al. Comparison of clinical features of acute lower respiratory tract infections in infants with RSV/HRV infection, and incidences of subsequent wheezing or asthma in childhood. BMC Infect Dis 2020;20:387. [Crossref] [PubMed]
- Bizzintino J, Lee WM, Laing IA, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 2011;37:1037-42. [Crossref] [PubMed]
- Huang HS, Tsai CL, Chang J, et al. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis. Clin Microbiol Infect 2018;24:1055-63. [Crossref] [PubMed]
- Zhong P, Zhang H, Chen X, et al. Clinical characteristics of the lower respiratory tract infection caused by a single infection or coinfection of the human parainfluenza virus in children. J Med Virol 2019;91:1625-32. [Crossref] [PubMed]
- Sricharoenchai S, Palla E, Sanicas M. Seasonality of Respiratory Syncytial Virus - Lower Respiratory Tract Infection (RSV-LRTI) in Children in Developing Countries. J Hum Virol Retrovirol 2016;3:00076.
- Crofts KF, Alexander-Miller MA. Challenges for the Newborn Immune Response to Respiratory Virus Infection and Vaccination. Vaccines (Basel) 2020;8:558. [Crossref] [PubMed]
- Sebina I, Phipps S. The Contribution of Neutrophils to the Pathogenesis of RSV Bronchiolitis. Viruses 2020;12:808. [Crossref] [PubMed]
- Petrarca L, Nenna R, Frassanito A, et al. Acute bronchiolitis: Influence of viral co-infection in infants hospitalized over 12 consecutive epidemic seasons. J Med Virol 2018;90:631-8. [Crossref] [PubMed]
- Li K, Peng YG, Yan RH, et al. Age-dependent changes of total and differential white blood cell counts in children. Chin Med J (Engl) 2020;133:1900-7. [Crossref] [PubMed]
- Dogru M, Yesiltepe Mutlu RG. The evaluation of neutrophil-lymphocyte ratio in children with asthma. Allergol Immunopathol (Madr) 2016;44:292-6. [Crossref] [PubMed]
- Resch B. Burden of respiratory syncytial virus infection in young children. World J Clin Pediatr 2012;1:8-12. [Crossref] [PubMed]
- Mohapatra SS, Boyapalle S. Epidemiologic, experimental, and clinical links between respiratory syncytial virus infection and asthma. Clin Microbiol Rev 2008;21:495-504. [Crossref] [PubMed]
- Stokes KL, Currier MG, Sakamoto K, et al. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. J Virol 2013;87:10070-82. [Crossref] [PubMed]
- Al Shibli A, Nouredin MB, Al Amri A, et al. Epidemiology of Bronchiolitis in Hospitalized Infants at Tawam Hospital, Al Ain, United Arab Emirates. Open Respir Med J 2021;15:7-13. [Crossref] [PubMed]
- Gregory SM, Nazir SA, Metcalf JP. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virol 2011;6:357-74. [Crossref] [PubMed]
- Han H, Xu Z, Cheng X, et al. Descriptive, Retrospective Study of the Clinical Characteristics of Asymptomatic COVID-19 Patients. mSphere 2020;5:e00922-20. [Crossref] [PubMed]


