
A case report of Prader-Willi syndrome in a child with metabolic disorders and severe obstructive sleep apnea treated effectively with continuous positive airway pressure
Highlight box
Key findings
• We report a case of Prader-Willi syndrome (PWS) with severe obstructive sleep apnea in a child with contraindications to growth hormone, who had a significant improvement in height after continuous positive airway pressure (CPAP) therapy alone, but the diagnostic delay is noteworthy.
What is known and what is new?
• PWS is a rare genetic disease, and early diagnosis and treatment are necessary.
• In this case, the patient’s height improved after CPAP treatment alone, but due to differences in understanding of the disease, the diagnosis and treatment of the patient were delayed.
What is the implication, and what should change now?
• There are also differences in the cognition of diseases, especially rare diseases, among doctors at different levels. The health education of genetic diseases should be strengthened, doctors should improve their diagnosis and treatment ability, and any special differences in signs should not be ignored.
Introduction
Prader-Willi syndrome (PWS) is a rare genetic disease with an incidence of 1/10,000–1/30,000. The main manifestations are hypotonia, disability, delayed gonadal development, and obesity (1). The main causes are paternal chromosome 15q11.2-13 gene expression defects and maternal haploidy (2). Children with PWS have endocrine and metabolic disorders such as feeding difficulties, short stature, hypogonadism, progressive obesity, breathing and sleep disorders, and diabetes (3). The incidence of sleep apnea syndrome (SAS) in children with PWS is less than 30% (4). At present, SAS in children is believed to be caused by a variety of factors including obesity, hypotonia, and craniocerebral skeletal deformities. Adenoid and tonsil hypertrophy can also lead to sleep disturbance (5-7).
We describe a case of PWS in a child with metabolic disorder and severe SAS, in which the patient was unable to use growth hormone. Therefore, we started continuous positive airway pressure (CPAP) treatment, after which, the patient’s SAS improved significantly, and a height increase of 4 cm was observed at 1 year. We present this case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-537/rc).
Case presentation
A 13-year-old presented to the hospital with lethargy, ptosis, and sudden weight gain. He was born crying without sound and had difficulty with sucking. He was unable to sit independently at 6 months of age and unable to walk until 2 years of age. He had poor academic performance, reduced physical activity, and a stubborn personality. He had been diagnosed with cryptorchidism at the age of 2 years and had undergone orchiopexy.
On admission, his height was 142 cm, weight 79 kg, and body mass index (BMI) was 39.2 kg/m2. The patient had an obese body type, moon face, and acanthosis nigricans on the posterior neck. Genital development was delayed and muscle tone decreased. The results of laboratory examination were abnormal (Table 1). Laboratory examination results suggested that the child had metabolic disorders. After admission, the child was found to have complete growth hormone deficiency, hypothyroidism, and a significantly lower bone age than children of the same age. Imaging examination showed that the lumbar spine was curved to the left (Figure 1). The patient had daytime sleepiness and sleep apnea at night. Polysomnography (PSG) monitoring was performed in the sleep medicine center, and the results showed 127 obstructive pauses, 9 central pauses, 20 mixed pauses, and 142 instances of hypopnea (Table 2). The patient had disordered sleep structure, NREM 1 stage accounted for 6.5%, NREM 2 stage accounted for 33.4%, and NREM 3 stage accounted for 42.9%. The apnea-hypopnea index (AHI) was 36.6 events/hour. The mean nocturnal oxygen saturation was 92%, the lowest pulse oxygen saturation was 57%, and the patient received a diagnosis of severe SAS and hypoxemia (Figure 2).
Table 1
| Item | Results | Reference range |
|---|---|---|
| Thyroid stimulating hormone, mIU/L | 4.41 ↑ | 0.28–4.3 |
| Free thyroxine, pmol/L | 12.79 ↓ | 13.9–22.1 |
| Testosterone, nmol/L | 0.96 ↓ | 9.9–27.8 |
| Uric acid, μmol/L | 468.4 ↑ | 208–428 |
| Total cholesterol, mmol/L | 5.58 ↑ | 0–5.1 |
| Low-density lipoprotein, mmol/L | 3.58 ↑ | ≤3.37 |
| High-density lipoprotein, mmol/L | 1.1 | 1.16–1.42 |
| Triglyceride, mmol/L | 1.76 ↑ | 0–1.6 |
| Fasting insulin, mIU/L | 21.8 ↑ | 3–17 |
Table 2
| Item | PSG | PSG + CPAP |
|---|---|---|
| Mean SpO2 (%) | 92 | 96 |
| AHI (events/hour) | 36.6 | 8.5 |
| SpO2 <90%, % TST | 17.4 | 1.1 |
| Respiratory arousal index | 1.4 | 0.2 |
| Sleep apnea (events) | ||
| Sleep | 156 | 50 |
| REM | 79 | 25 |
| NREM | 77 | 25 |
| OSA | 127 | 15 |
| CSA | 20 | 1 |
| MSA | 9 | 33 |
| HS (events) | 142 | 6 |
| Proportion of sleep stage (%) | ||
| REM | 17.2 | 16.1 |
| NREM 1 | 6.5 | 2.8 |
| NREM 2 | 33.4 | 38.3 |
| NREM 3 | 42.9 | 42.8 |
AHI, apnea hypopnea index; CPAP, continuous positive airway pressure; CSA, central sleep apnea; HS, hypopnea; MSA, mixed sleep apnea; NREM, non-rapid eye movement sleep; OSA, obstructive sleep apnea; PSG, polysomnography; REM, rapid eye movement; SpO2, peripheral oxygen saturation; TST, total sleep time.
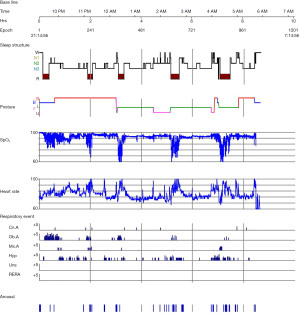
All examination results showed metabolic disorders, developmental failure, and severe obstructive sleep apnea (OSA). We performed chromosome karyotyping and genetic testing, and the karyotype was 46, XY, 14ps+. Multiplex ligation-dependent probe amplification gene analysis detected gene deletion and abnormal methylation in the 15q11-13 region of the patient. We diagnosed PWS based on the history and examination results.
Although the patient had sleep disturbances and metabolic disorders, his obesity, diabetes, and severe sleep apnea were contraindications to growth hormone therapy (8-10). Therefore, the patient underwent weight loss and CPAP therapy, with close monitoring of glycemic changes and height changes. The lowest and highest CPAP pressures were 4 and 14 cmH2O, respectively. The mean oxygen saturation after treatment was 97%. After the current CPAP treatment, the child had a significant decrease in SAS (Table 2), good glycemic control, and a 4-cm increase in height at 1 year. At present, his parents are willingly accepting of CPAP treatment and are satisfied with the treatment effect. The patient’s OSA symptoms are now significantly improved, and growth hormone therapy has been started after the doctor’s evaluation.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was provided by the patient’s guardians for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
In this case, we report the case of a child with PWS with metabolic disorders and severe OSA. The patient had contraindications to growth hormone use (including severe OSA and insulin resistance). CPAP and weight-loss therapy were administered. CPAP treatment was very effective, and the patient had a significant decrease in AHI and a 4-cm increase in height at 1 year. There are few reports of improvement in height in PWS patients treated with CPAP alone.
Sleep disorders are common in children with PWS, and many studies have reported sleep disorders in PWS, but few studies have analyzed PSG results and CPAP treatment results in children with contraindications to growth hormone use. Xiao et al. reported a case of a child who underwent corrective oral surgery and CPAP treatment with a significant decrease in AHI after treatment, which was closely related to the treatment of both surgery and CPAP (11). Weselake et al. reported a significant decrease in AHI in a patient with PWS who was treated with modafinil and CPAP (12). Blecher et al. reported a case of a child with severe OSA and tonsillar hypertrophy secondary to the initiation of growth hormone after pharyngoplasty, who was eventually treated with CPAP with good response (13). Yanyu et al. reported a PWS patient with central sleep apnoea (CSA) who showed significant improvement in symptoms after CPAP treatment (14). Miller et al.’s study demonstrated that the use of growth hormone causes OSA in patients (15). Khayat et al.’s study showed that patients with CSA need continuous PSG monitoring, because some children with CSA will persist and have a risk of developing OSA (16). Shaikh et al. reported the need for a multidisciplinary approach during the transition from children with PWS to adults (17). The above reports indicate that the related factors of SAS in children with PWS are related to obesity, growth hormone treatment, and structure. At the same time, CPAP treatment has a positive effect on the improvement of quality of life in PWS patients. It is worth noting that PWS requires growth hormone therapy, but the use of growth hormone at the wrong time not only does not help the patient, but even has a negative effect on the patient.
The patient was a school-aged child with typical physical features of PWS, but the best opportunity for growth hormone treatment had been missed due to delayed diagnosis. PSG monitoring showed that the child had both obstructive and central apnea events, AHI was measured at 36.6, which indicated severe OSA and severe hypoxemia, which was not suitable for growth hormone treatment. At the same time, apnea events were significantly reduced after CPAP treatment. Ventilator therapy, weight loss, and correction of deformities such as scoliosis are active treatment options when growth hormone is contraindicated. At present, the patient has lost significant weight. After CPAP treatment, the OSA symptoms were improved, and further treatment with growth hormone was started.
In this case, we have gained valuable insights. Despite the patient exhibiting early symptoms of PWS, the diagnosis was significantly delayed. This delay can be attributed to disparities in medical education levels and disease awareness across different regions. Additionally, the varying levels of expertise among healthcare professionals, particularly in recognizing rare diseases, further contributed to the diagnostic challenges. To address these issues, it is crucial for society to enhance public education and awareness about genetic disorders. Medical practitioners must also strive to improve their diagnostic and treatment capabilities, paying close attention to any unusual clinical signs. Families should seek prompt medical attention when a child presents with atypical symptoms to ensure timely intervention and to safeguard the child’s health and quality of life.
Conclusions
This case reports a patient with PWS with severe OSA who had improved height after CPAP treatment alone. It is noteworthy that the diagnostic delay in this case is worthy of consideration. Due to the differences in medical education and medical level, it is an important reason for the delay of diagnosis. Any signs of special differences should not be ignored. Timely diagnosis and treatment can improve the quality of life of children and strengthen the education of genetic diseases.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-537/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-537/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-537/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was provided by the patient’s guardians for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mas-Parés B, Carreras-Badosa G, Gómez-Vilarrubla A, et al. Sex dimorphic associations of Prader-Willi imprinted gene expressions in umbilical cord with prenatal and postnatal growth in healthy infants. World J Pediatr 2025;21:100-12. [Crossref] [PubMed]
- Godler DE, Singh D, Butler MG. Genetics of Prader-Willi and Angelman syndromes: 2024 update. Curr Opin Psychiatry 2025;38:95-100. [Crossref] [PubMed]
- Qiu L, Chang A, Ma R, et al. Neuromodulation for the treatment of Prader-Willi syndrome - A systematic review. Neurotherapeutics 2024;21:e00339. [Crossref] [PubMed]
- Vandeleur M, Davey MJ, Nixon GM. Are sleep studies helpful in children with Prader-Willi syndrome prior to commencement of growth hormone therapy? J Paediatr Child Health 2013;49:238-41. [Crossref] [PubMed]
- Duis J, Pullen LC, Picone M, et al. Diagnosis and management of sleep disorders in Prader-Willi syndrome. J Clin Sleep Med 2022;18:1687-96. [Crossref] [PubMed]
- Cataldi M, Arnaldi D, Tucci V, et al. Sleep disorders in Prader-Willi syndrome, evidence from animal models and humans. Sleep Med Rev 2021;57:101432. [Crossref] [PubMed]
- Itani R, Gillett ES, Perez IA. Sleep Consequences of Prader-Willi Syndrome. Curr Neurol Neurosci Rep 2023;23:25-32. [Crossref] [PubMed]
- Tauber M, Hoybye C. Endocrine disorders in Prader-Willi syndrome: a model to understand and treat hypothalamic dysfunction. Lancet Diabetes Endocrinol 2021;9:235-46. [Crossref] [PubMed]
- Presti S, Pavone M, Verrillo E, et al. Long Term Ventilation in Pediatric Central Apnea: Etiologies and Therapeutic Approach over a Decade. Pediatr Pulmonol 2025;60:e27400. [Crossref] [PubMed]
- Bamba V, Kanakatti Shankar R. Approach to the Patient: Safety of Growth Hormone Replacement in Children and Adolescents. J Clin Endocrinol Metab 2022;107:847-61. [Crossref] [PubMed]
- Xiao KK, Tomur S, Beckerman R, et al. Orthognathic Correction in Prader-Willi Syndrome: Occlusion and Sleep Restored. Cleft Palate Craniofac J 2019;56:415-8. [Crossref] [PubMed]
- Weselake SV, Foulds JL, Couch R, et al. Prader-Willi syndrome, excessive daytime sleepiness, and narcoleptic symptoms: a case report. J Med Case Rep 2014;8:127. [Crossref] [PubMed]
- Blecher G, Wainbergas N, McGlynn M, et al. Rapidly evolving narcolepsy-like syndrome coinciding with severe OSA following pharyngoplasty in Prader-Willi syndrome. Respirol Case Rep 2014;2:111-2. [Crossref] [PubMed]
- He Y, Wang Y, Hao C, et al. Prader-Willi syndrome with severe sleep apnea syndrome and obesity hypoventilation syndrome: a case report and literature review. Journal of Clinical Pediatrics 2019;37:927-31.
- Miller J, Silverstein J, Shuster J, et al. Short-term effects of growth hormone on sleep abnormalities in Prader-Willi syndrome. J Clin Endocrinol Metab 2006;91:413-7. [Crossref] [PubMed]
- Khayat A, Narang I, Bin-Hasan S, et al. Longitudinal evaluation of sleep disordered breathing in infants with Prader-Willi syndrome. Arch Dis Child 2017;102:634-8. [Crossref] [PubMed]
- Shaikh MG, Barrett TG, Bridges N, et al. Prader-Willi syndrome: guidance for children and transition into adulthood. Endocr Connect 2024;13:e240091. [Crossref] [PubMed]



