
Age- and sex-specific reference intervals for anti-Müllerian hormone during childhood in Chinese population: a cross-sectional study of 2,450 healthy children
Highlight box
Key findings
• This study established age- and sex-specific serum anti-Müllerian hormone (AMH) reference intervals (RIs) for children in Wuhan, China.
What is known and what is new?
• AMH serves as a reliable biomarker of testicular function in males and ovarian function in females, and the application of AMH in the diagnosis of pediatric endocrine disorders is becoming increasingly prevalent.
• AMH exhibits significant fluctuations across the various stages of growth and development in both genders. This analysis examined the age- and sex-specific RIs for serum AMH in 2,450 healthy children from Wuhan, China, ranging from 1 day to 19 years old. The RIs for serum AMH established using the Mindray CL6000i system were relatively reliable and may demonstrate sufficient comparability across different instruments.
What is the implication, and what should change now?
• This study established age- and sex-specific RIs for AMH on the Mindray CL-6000i platform, facilitating effective laboratory use. The RIs can augment the clinical application of AMH measurement in pediatric care.
Introduction
Anti-Müllerian hormone (AMH), alternatively termed Müllerian-inhibiting substance, is a 140-kDa homodimeric glycoprotein belonging to the transforming growth factor (TGF)-β superfamily (1,2). This hormone not only plays a pivotal role in regulating embryonic sex differentiation by controlling the involution of the Müllerian ducts (3) but also exerts a profound influence on both male testicular and female ovarian function (4,5). Significantly, AMH exhibits fluctuations across the various stages of growth and development in both genders (6). Hence, timely and accurate monitoring of AMH levels is crucial for clinical decision-making regarding management of growing and developing children, as well as the related and associated diseases.
AMH is generally considered to be closely related to the number of primordial follicles and has been traditionally used to assess female ovarian function and fertility (7,8). For instance, AMH has been clinically applied in the evaluation of ovarian reserve, the diagnosis of polycystic ovary syndrome (PCOS), and the prediction of ovarian response to hyperstimulation during in vitro fertilization (IVF) in adults (9,10). Moreover, it is particularly noteworthy that AMH is also associated with the sexual development process in children, which is regulated by the hypothalamic-pituitary-gonadal (HPG) axis. Abnormal changes in AMH levels may indicate the presence of sexual development-related diseases in children, such as precocious or delayed puberty (11). Compared to the relatively inactive testosterone and gonadotropin secretion before puberty, AMH can also reflect testicular function. Low or undetectable AMH concentrations can indicate primary testicular dysfunction, which is commonly observed in patients with conditions such as cryptorchidism, monorchidism, partial gonadal dysplasia, or central hypogonadism. Therefore, AMH is an invaluable biomarker for achieving adequate diagnosis while avoiding the need for invasive surgical procedures (12-14). The serum AMH level in male children with precocious puberty is lower and can return to prepubertal levels after successful treatment. Conversely, if the serum AMH level remains high during puberty, it may indicate delayed puberty or the presence of sex cord-stromal tumors (15).
In addition, AMH can serve as a marker of gonadotoxicity (16). Hematopoietic stem cell transplantation and cytotoxic treatments such as chemotherapy can easily lead to gonadal dysfunction and premature ovarian failure in children and adolescents. Compared to other sex hormones, such as follicle-stimulating hormone (FSH), AMH offers an earlier prediction of the onset of ovarian failure. This allows the determination of the need for ovarian tissue cryopreservation and transplantation as a means to maintain fertility (17-20). Precise diagnostics depend on establishing reference intervals (RIs). Thus, establishing the RIs of AMH and timely identification of abnormal changes have key clinical application value for monitoring sexual development-related diseases in children and assessing fertility.
In recent years, the establishment of RIs for AMH in adult female populations across Western and Asian countries has significantly broadened its clinical applications, particularly as a diagnostic tool for PCOS (21). However, notable differences exist in AMH level trends between children and adults. The direct application of adult AMH RIs to children may not accurately reflect children’s physiological status and ovarian function, thereby limiting its clinical utility in pediatrics. AMH serves as a reliable biomarker for assessing testicular and ovarian function in children and adolescents, necessitating the establishment of RI tailored to children based on gender and age. Unfortunately, data on serum AMH levels in individuals aged 1 day to 19 years remain scarce to the best of our knowledge. This inadequately captures the dynamic changes in AMH levels during childhood and adolescence, further complicating the accurate interpretation of AMH results in pediatric patients. Consequently, there is an urgent need to re-establish the RIs of AMH for specific age groups using large, representative cohorts.
The establishment of RIs is contingent upon accurate and reliable AMH testing. The AMH Gen II ELISA assay supplanted the cumbersome first-generation AMH assay and gained widespread use. However, its reliability has been questioned due to its poor reproducibility. Subsequently developed automated chemiluminescent immunoassays (CLIAs) have addressed these deficiencies, further enhancing reproducibility and accuracy (2). Prominent automated immunoassay platforms include those offered by Beckman Coulter and Roche. A study has attested to the exceptional precision, sensitivity, linearity, and specificity of the CLIAs from Mindray (Shenzhen, China), which exhibit high concordance with Roche’s enhanced CLIA results (22).
This study aimed to systematically establish age- and gender-specific RIs for AMH in healthy Chinese children based on previous cohorts (23,24) using the Mindray CL-6000i automatic chemiluminescence immunoassay analyzer. The ultimate goal was to augment the clinical application of AMH measurement in pediatric care. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-104/rc).
Methods
Research participant recruitment
In total, 2,506 healthy pediatric participants were recruited from the Physical Examination Center at Wuhan Children’s Hospital between September 2022 and August 2023. The inclusion criteria were as follows: healthy full-term neonates with birth weight ranging from 2.5 to 4.0 kg, an Apgar score of 8 to 10; and born without apparent congenital anomalies, genetic disorders. For healthy newborns, children, and adolescents, physical examination and laboratory test results were required to be within the reference range. Meanwhile, the exclusion criteria were as follows: suspicion of disorders of sex development (DSD), Turner syndrome, or PCOS; premature ovarian insufficiency; inherited metabolic disease; hepatorenal dysfunction; circulatory system disease; acute or chronic infection; and prescribed or non-prescribed medications for endocrine disease. Children with blood specimen abnormalities, such as hemolysis, were also excluded (Figure 1). After screening, 2,450 samples from healthy individuals (aged 1 day to 19 years) were included in the establishment of the RI for AMH. Participants were stratified into nine age-specific groups based on age distribution: 1 day–1 month, >1–12 months, >1–3 years, >3–6 years, >6–9 years, >9–12 years, >12–15 years, >15–18 years, and 18–19 years.

Ethical approval
The cross-sectional study adhered to the 2013 revision of the Declaration of Helsinki and received approval from the Ethics Committee of Wuhan Children’s Hospital (No. 2024R042-E01). Written informed consent was obtained from all participants or their legal guardians (at least one guardian).
Sample collection and measurement
Venous blood (approximately 1–4 mL) was drawn in the morning from each participant when they most likely had an empty stomach. As in our previous study (23), the serum was processed and separated within 6 hours using the Roche Cobas P612 preprocessing system (Roche Diagnostics, Basel, Switzerland). Serum AMH levels were quantified using the Mindray CL-6000i automated CLIA analyzer. Standard methodologies were employed, dedicated reagents (Mindray) were used according to the manufacturer’s instructions, and routine maintenance was performed. The qualified serum samples were stored at −80 ℃ prior to analysis.
Quality control
In a previous study (22), the analytical and clinical diagnostic performance of AMH assays were thoroughly evaluated. All tests were performed according to standard operating procedures recommended by the National Committee for Clinical Laboratory Standards (NCCLS). The analytical procedure was rigorously governed by regular maintenance, calibration, and quality control according to the Mindray manufacturer's instructions, clinical laboratory norms, and standard operating procedures. Sample analysis proceeded only when all analytical parameters were acceptable. The linear range of the AMH kit was 0.01–23 ng/mL, and the limit of detection (LoD) for this assay was 0.01 ng/mL.
Statistical analysis
The dataset was generated using R v. 3.6.3 and R-Studio v, 1.4.1106 (The R Foundation for Statistical Computing, Vienna, Austria). The categorical variables are expressed as frequencies and percentages. Outliers were removed following the Clinical and Laboratory Standards Institute (CLSI) EP9-A3 guidelines via a combination of the generalized extreme studentized deviation (ESD) method and box plots (≤5% outlier ratio). Data normality was assessed with the Shapiro-Wilk test and skewness/kurtosis analysis. Sex-related differences in AMH distribution were analyzed using the Kruskal-Wallis test. Probability density curves for AMH in males, females, and the total population were plotted using the “ggplot2” package in R. A scatter plot and linear regression of AMH levels across ages was created in GraphPad Prism version 8.00 (GraphPad Software, Dotmatics, Boston, MA, USA). When comparing AMH values across different genders and age groups, adjacent age groups were merged if no significant differences were observed between them. Piecewise regression analysis was used to explore the changing relationship of AMH distribution at different age stages, so as to determine the optimal age range for AMH distribution among different gender populations AMH levels were reported as the 2.5th (P2.5), 50th (P50), and 97.5th (P97.5) percentiles. The RI was with P2.5 as the lower limit and P97.5 as the upper limit. All P values were two sided, with P<0.05 considered statistically significant. Spearman correlation coefficients were calculated to evaluate the correlation strength between serum AMH levels and estradiol (E2), FSH, luteinizing hormone (LH), prolactin (PRL), progesterone (PROG), and testosterone (TESTO).
Results
Characteristics of the study population
Based on the generalized ESD method and box diagram, 21 AMH values were eliminated as outliers. A total of 2,450 healthy individuals aged 1 day–19 years in the study, including 1,031 females and 1,419 males, were enrolled and stratified into age subgroups. Among the participants, 167 (female 6.69%; male 6.91%) were aged under 1 month (newborn), 223 (female 7.76%; male 10.08%) were aged >1–12 months (infant), 140 (female 6.79%; male 4.93%) were aged >1–3 years, 209 (female 10.28%; male 7.26%) were aged >3–6 years, 583 (female 25.8%; male 22.34%) were aged >6–9 years, 417 (female 16.68%; male 17.27%) were aged >9–12 years, 216 (female 10.09%; male 7.89%) were aged >12–15 years, 227 (female 11.64%; male 7.54%) were aged >15–18 years, and 268 (female 4.27%; male 15.79%) were aged >18–19 years. Detailed characteristics of the study populations were shown in Table 1.
Table 1
| Age group | Outlier | Total | Female | Male |
|---|---|---|---|---|
| 1 day–1 month | 3 (1.76) | 167 | 69 (6.69) | 98 (6.91) |
| >1–12 months | 0 | 223 | 80 (7.76) | 143 (10.08) |
| >1–3 years | 0 | 140 | 70 (6.79) | 70 (4.93) |
| >3–6 years | 0 | 209 | 106 (10.28) | 103 (7.26) |
| >6–9 years | 2 (0.34) | 583 | 266 (25.8) | 317 (22.34) |
| >9–12 years | 3 (0.71) | 417 | 172 (16.68) | 245 (17.27) |
| >12–15 years | 8 (3.57) | 216 | 104 (10.09) | 112 (7.89) |
| >15–18 years | 4 (1.73) | 227 | 120 (11.64) | 107 (7.54) |
| >18–19 years | 1 (0.37) | 268 | 44 (4.27) | 224 (15.79) |
| 1 day–19 years | 21 (0.85) | 2,450 | 1,031 (100.00) | 1,419 (100.00) |
Data are presented as n (%).
Distribution characteristics of AMH
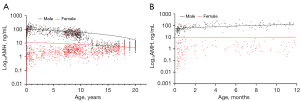
The probability density distributions of serum AMH levels across the total population, males, and females were presented in Figure 2. The distribution of serum AMH levels in the pediatric population was markedly skewed by Shapiro-Wilk test (W=0.015, P<0.01). The median and 95% interval range (P2.5–P97.5) of AMH levels, categorized by age and sex, were displayed in Table 2. The overall serum median of AMH was 6.21 (95% interval range, 0.44–137.22) ng/mL. This was higher in males (median 33.45 ng/mL; 95% interval range, 1.71–155.83 ng/mL) than in females (median 2.48 ng/mL; 95% interval range, 0.11–80.57 ng/mL) (P<0.001; Table 2). In males, the levels were highly variable in infants under 1 year of age, with a gradual increase as age progressed from 1 to 12 months. Conversely, in females, the variation was minimal (Figure 3).
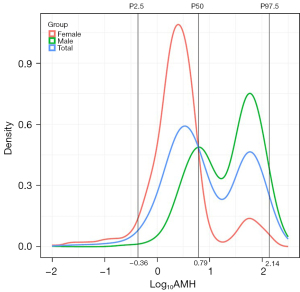
Table 2
| Age group | Total | Male | Female | P |
|---|---|---|---|---|
| 1 day–1 month | 27.15 (0.02, 112.04) | 46.49 (2.89, 120.15) | 0.27 (0.01, 88.7) | <0.001 |
| >1–12 months | 62.42 (0.28, 224.26) | 94.75 (0.49, 248.73) | 1.43 (0.16, 152.77) | <0.001 |
| >1–3 years | 6.04 (0.35, 208.01) | 91.9 (3.54, 211.68) | 1.33 (0.26, 80.51) | <0.001 |
| >3–6 years | 11.8 (0.54, 136.52) | 74.27 (2.52, 157.01) | 2.17 (0.48, 71.87) | <0.001 |
| >6–9 years | 24 (0.99, 108.44) | 45.28 (1.03, 113.16) | 3.23 (0.89, 102.78) | <0.001 |
| >9–12 years | 19.77 (0.85, 93.67) | 39.18 (2.99, 102) | 2.75 (0.58, 73.94) | <0.001 |
| >12–15 years | 3.77 (0.67, 15.16) | 6.22 (1.4, 17.03) | 2.13 (0.42, 7.1) | <0.001 |
| >15–18 years | 4.19 (1.09, 12.82) | 5.33 (0.83, 13.72) | 3.41 (1.12, 8.9) | <0.001 |
| >18–19 years | 6.11 (1.77, 16.44) | 6.79 (2.22, 17.32) | 3.46 (0.82, 13.37) | <0.001 |
| 1 day–19 years | 6.21 (0.44, 137.22) | 33.45 (1.71, 155.83) | 2.48 (0.11, 80.57) | <0.001 |
Data were analyzed by the Kruskal-Wallis test and are presented as P50 (P2.5–P97.5). AMH, anti-Müllerian hormone.
Pediatric RIs
Serum AMH levels across different age groups were analyzed using the Kruskal-Wallis test. By consolidating adjacent age groups without significant differences from Table 2, the age- and sex-specific pediatric RIs for AMH were shown in Figure 4 and Table 3. In males, serum AMH levels were high at birth; increased markedly from 1 to 12 months, with a P50 of 94.75 (95% interval range, 0.49–248.73) ng/mL; continuously declined until 18 years of age, with a notable decrease observed between 12 and 15 years (P50 6.22 ng/mL; 95% interval range, 1.4–17.03 ng/mL), followed by a slight increase between 18 and 19 years. In females, serum AMH levels were low within the first month after birth, with a P50 of 0.27 (95% interval range, 0.01–88.7) ng/mL, gradually rose thereafter, peaked at 9 years, underwent a slight decline between 9 and 15 years, and reached a plateau at 15 to 19 years. Compared to males of corresponding ages, females demonstrated substantially lower AMH levels, especially in the neonates.
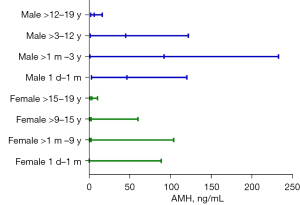
Table 3
| Group | RI, P50 (P2.5, P97.5) |
|---|---|
| Male | |
| 1 day–1 month | 46.49 (2.89, 120.15) |
| >1 month–3 years | 92.20 (1.05, 232.77) |
| >3–12 years | 44.97 (1.50, 121.97) |
| >12–19 years | 6.23 (1.94, 16.14) |
| Female | |
| 1 day–1 month | 0.27 (0.01, 88.7) |
| >1 month–9 years | 2.32 (0.45, 103.99) |
| >9–15 years | 2.49 (0.51, 60.00) |
| >15–19 years | 3.44 (1.13, 10.32) |
AMH, anti-Müllerian hormone; P2.5, 2.5th percentile; P50, median; P97.5, 97.5th percentile; RI, reference interval.
Correlation between AMH and six sex hormones
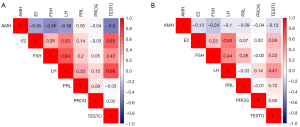
In males, the correlation heatmap revealed weak negative correlations between AMH and LH (r=–0.38) and E2 (r=–0.35); meanwhile, AMH was moderately negatively correlated with FSH (r=–0.46) and TESTO (r=–0.5). In females, a weak negative correlation was observed between AMH and FSH (r=–0.24) (Figure 5).
Discussion
This study represents the largest investigation to date to establish age- and sex-specific RIs for AMH in Chinese children aged 1 day to 19 years, a period widely recognized as crucial for of gonadal development. Recent guideline (25) also indicate that AMH RIs in clinical laboratories can serve as a critical tool for clinicians in accurately interpreting patient results, significantly enhancing the application of AMH in the diagnosis of pediatric endocrine disorders.
Notably, we observed high concentrations of AMH in males at birth, with levels peaking between 1 months and 1 year of age, subsequently declining significantly with advancing age, and being at the low level in adolescence. This pattern aligns with previous research findings (26). The surge in AMH during male infancy is attributed to minipuberty, a transient period which is crucial for sexual development and is characterized by heightened HPG axis activity (27). Within the first week after birth until approximately 6 months of age, males experience an increase in gonadotropin secretion, including LH and FSH. FSH stimulates the production of testicular AMH through the induction of Sertoli cell proliferation, and the upregulation of AMH transcription and AMH production during this phase is not modulated by androgen negative feedback, leading to exceptionally high AMH levels. During puberty, the increase in TESTO concentration and the maturation of Sertoli cells lead to the downregulation of AMH expression (28). In contrast, female AMH levels in our study were low at birth but gradually increased during infancy and childhood. Later, they rose slowly, reaching a moderate peak around the age of 9 years and further increased between the ages of 15 and 19 years. Yates et al. argued that the increase of AMH levels in the female age group of 8–12 years might indicate hormonal changes at the onset of puberty (29). These observations also align with previously published data, underscoring the reliability of our study results (30). Additionally, compared to our research, the P97.5 AMH levels in females reported by Jopling et al. were relatively lower (31), which may be related to ethnicity, as corresponding studies have found that AMH levels tend to be lower in black and Hispanic women, while Chinese women under the age of 25 generally have significantly higher AMH levels than Caucasian women (32). In summary, when it comes to AMH reference ranges, there are many factors that influence AMH test values, including gender, ethnicity, geographic location, and testing equipment systems, all of which need to be considered in the analysis of test results.
The Mindray CLIA analyzer demonstrated a high degree of concordance with the Roche Cobas E602 analyzer in AMH detection (22), exhibiting high precision and accuracy, which are essential for establishing robust RIs. In this study, we achieved a more comprehensive and detailed stratification by age and gender through the inclusion of 2,450 participants, thereby establishing more precise RIs of AMH in Chinese children. Conversely, previous studies have often been limited to selected pediatric populations, which potentially compromise the generalizability of the established RIs due to factors such as small sample sizes and the lack of specific stratifications by age and gender. For example, Ronn et al. established RIs of AMH with the Beckman assay based on serum samples from 300 healthy girls aged 6 to 19 years within the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) cohort (33). Additionally, some studies still employed first-generation AMH detection methods (34,35).
To better understand the role of AMH in sexual development, we analyzed the correlation between AMH and six sex hormones based on our previous research (36). We found that in healthy males, AMH exhibited moderate negative correlations with FSH and TESTO, whereas in healthy females, AMH demonstrated a weak negative correlation with FSH. In males, as previously discussed, the upregulation of AMH can be explained by two mechanisms: the first is the direct effect of FSH on Sertoli cell proliferation, and the second is the direct action on AMH transcription. Interestingly, during normal puberty, FSH and AMH levels are negatively correlated due to TESTO’s potent inhibition of AMH expression, overriding FSH stimulation (37). For females, evidence indicates a negative correlation between AMH and FSH levels during controlled ovarian stimulation with FSH (10). The reason may be that FSH is a crucial hormone in follicular development, promoting the growth and maturation of follicles; as its levels rise, more follicles are recruited into the growth cycle, leading to the depletion of ovarian reserve and a subsequent reduction in the number of follicles producing AMH (38). Additionally, research indicates that TESTO exerts a negative regulatory effect on AMH (14,28), which is consistent with the results we observed in male patients in our study. Another recent study demonstrated that AMH exerts pivotal functions within the HPG axis, including the modulation of gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus, gonadotropin secretion in the pituitary gland, and gonadal function (39). These findings constitute novel insights into the regulatory mechanisms of reproductive endocrinology. These sex hormones are derived from the HPG axis (FSH, LH, and PRL are produced by the pituitary, and E2, PROG and TESTO are produced by the gonads). Thus, we speculate that AMH may be linked to the HPG axis and further validation is required with larger population-based studies.
The RIs established in this study are highly relevant to the clinical practice of pediatric endocrinology. By providing accurate AMH reference values for different age groups and genders, clinicians can better assess the gonadal function and development of pediatric patients. This is particularly useful in the diagnosis of DSD, precocious puberty, and delayed puberty. Additionally, the RIs can guide the monitoring of gonadal function in pediatric cancer survivors undergoing gonadotoxic therapies.
Despite the strengths of our study, several limitations should be acknowledged. First, the sample size, while larger than that of some previous studies, may still be insufficient to fully capture the variability in AMH levels within each age and gender group. Future studies with even larger sample sizes are warranted to further validate and refine the RIs. Second, due to the unique characteristics of the pediatric population, sample collection proves challenging, and consequently, achieving a 1:1 male-to-female ratio in certain age groups is not feasible. Finally, our study was conducted at a single center, necessitating further validation through multicenter trials.
Conclusions
The gender- and age-specific RIs of AMH established in this study, which cover the complete range from 1 day to 19 years of age, represent an important contribution to the field of pediatric endocrinology. By enhancing the clinical application of AMH, these RIs have the potential to improve the diagnosis and management of pediatric endocrine disorders. Future research should focus on validating and refining these intervals in larger, more diverse populations and on clarifying the longitudinal changes in AMH levels throughout childhood and adolescence.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-104/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-104/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-104/prf
Funding: This work was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-104/coif). A.F.C. serves as an Editor-in-Chief of Translational Pediatrics from June 2023 to May 2025. L.L., C.Q., J.L., and W.L. are from Shenzhen Mindray Bio-Medical Electronics Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) for research on human participants and was reviewed and approved by the Ethics Committee of Wuhan Children’s Hospital (No. 2024R042-E01). Written informed consent was obtained from all participants or their legal guardians (at least one guardian).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howard JA, Hart KN, Thompson TB. Molecular Mechanisms of AMH Signaling. Front Endocrinol (Lausanne) 2022;13:927824. [Crossref] [PubMed]
- Li HWR, Robertson DM, Burns C, et al. Challenges in Measuring AMH in the Clinical Setting. Front Endocrinol (Lausanne) 2021;12:691432. [Crossref] [PubMed]
- Rey R, Josso N, Racine C. Sexual Differentiation. In: Feingold KR, Anawalt B, Blackman MR et al., editors. Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2024, MDText.com, Inc.; 2000.
- Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 2014;20:370-85. [Crossref] [PubMed]
- Rey RA, Grinspon RP. Anti-Müllerian hormone, testicular descent and cryptorchidism. Front Endocrinol (Lausanne) 2024;15:1361032. [Crossref] [PubMed]
- Jeffery A, Streeter AJ, Hosking J, et al. Anti-Müllerian hormone in children: a ten-year prospective longitudinal study (EarlyBird 39). J Pediatr Endocrinol Metab 2015;28:1153-62. [Crossref] [PubMed]
- Broer SL, Broekmans FJ, Laven JS, et al. Anti-Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update 2014;20:688-701. [Crossref] [PubMed]
- Moolhuijsen LME, Visser JA. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J Clin Endocrinol Metab 2020;105:3361-73. [Crossref] [PubMed]
- Bedenk J, Vrtačnik-Bokal E, Virant-Klun I. The role of anti-Müllerian hormone (AMH) in ovarian disease and infertility. J Assist Reprod Genet 2020;37:89-100. [Crossref] [PubMed]
- di Clemente N, Racine C, Pierre A, et al. Anti-Müllerian Hormone in Female Reproduction. Endocr Rev 2021;42:753-82. [Crossref] [PubMed]
- Xue J, Song W, Si M, et al. Serum Kisspeptin and AMH Levels Are Good References for Precocious Puberty Progression. Int J Endocrinol 2020;2020:3126309. [Crossref] [PubMed]
- Josso N, Rey RA, Picard JY. Anti-müllerian hormone: a valuable addition to the toolbox of the pediatric endocrinologist. Int J Endocrinol 2013;2013:674105. [Crossref] [PubMed]
- Lindhardt Johansen M, Hagen CP, Johannsen TH, et al. Anti-müllerian hormone and its clinical use in pediatrics with special emphasis on disorders of sex development. Int J Endocrinol 2013;2013:198698. [Crossref] [PubMed]
- Xu HY, Zhang HX, Xiao Z, et al. Regulation of anti-Müllerian hormone (AMH) in males and the associations of serum AMH with the disorders of male fertility. Asian J Androl 2019;21:109-14. [Crossref] [PubMed]
- Edelsztein NY, Grinspon RP, Schteingart HF, et al. Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int J Pediatr Endocrinol 2016;2016:20. [Crossref] [PubMed]
- Brougham MF, Crofton PM, Johnson EJ, et al. Anti-Müllerian hormone is a marker of gonadotoxicity in pre- and postpubertal girls treated for cancer: a prospective study. J Clin Endocrinol Metab 2012;97:2059-67. [Crossref] [PubMed]
- Abir R, Ben-Aharon I, Garor R, et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum Reprod 2016;31:750-62. [Crossref] [PubMed]
- Silber SJ, DeRosa M, Goldsmith S, et al. Cryopreservation and transplantation of ovarian tissue: results from one center in the USA. J Assist Reprod Genet 2018;35:2205-13. [Crossref] [PubMed]
- Hwang S, Lee Y, Yoon JH, et al. Long-term endocrine sequelae after hematopoietic stem cell transplantation in children and adolescents. Ann Pediatr Endocrinol Metab 2024;29:109-18. [Crossref] [PubMed]
- Lunsford AJ, Whelan K, McCormick K, et al. Antimüllerian hormone as a measure of reproductive function in female childhood cancer survivors. Fertil Steril 2014;101:227-31. [Crossref] [PubMed]
- Evliyaoglu O, Imöhl M, Weiskirchen R, et al. Age-specific reference values improve the diagnostic performance of AMH in polycystic ovary syndrome. Clin Chem Lab Med 2020;58:1291-301. [Crossref] [PubMed]
- Zhao JJ, Kang CM, Zhang P, et al. Performance characteristics of the Mindray chemiluminescence anti-Müllerian hormone assay. J Clin Lab Anal 2021;35:e23734. [Crossref] [PubMed]
- Cai Q, Liu M, Xie Z, et al. Specific reference interval for high-sensitivity cardiac troponin I among healthy children in Wuhan, China. Transl Pediatr 2024;13:908-20. [Crossref] [PubMed]
- Yao C, Wu M, Liu M, et al. Age- and sex-specific reference intervals for thyroid hormones in a Chinese pediatrics: a prospective observational study of 1,279 healthy children. Transl Pediatr 2021;10:2479-88. [Crossref] [PubMed]
- Ahmed SF, Achermann JC, Arlt W, et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015). Clin Endocrinol (Oxf) 2016;84:771-88. [Crossref] [PubMed]
- Aksglaede L, Sørensen K, Boas M, et al. Changes in anti-Müllerian hormone (AMH) throughout the life span: a population-based study of 1027 healthy males from birth (cord blood) to the age of 69 years. J Clin Endocrinol Metab 2010;95:5357-64. [Crossref] [PubMed]
- Grumbach MM. A window of opportunity: the diagnosis of gonadotropin deficiency in the male infant. J Clin Endocrinol Metab 2005;90:3122-7. [Crossref] [PubMed]
- Edelsztein NY, Valeri C, Lovaisa MM, et al. AMH Regulation by Steroids in the Mammalian Testis: Underlying Mechanisms and Clinical Implications. Front Endocrinol (Lausanne) 2022;13:906381. [Crossref] [PubMed]
- Yates AP, Jopling HM, Burgoyne NJ, et al. Paediatric reference intervals for plasma anti-Müllerian hormone: comparison of data from the Roche Elecsys assay and the Beckman Coulter Access assay using the same cohort of samples. Ann Clin Biochem 2019;56:536-47. [Crossref] [PubMed]
- Lie Fong S, Visser JA, Welt CK, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab 2012;97:4650-5. [Crossref] [PubMed]
- Jopling H, Yates A, Burgoyne N, et al. Paediatric Anti-Müllerian Hormone measurement: Male and female reference intervals established using the automated Beckman Coulter Access AMH assay. Endocrinol Diabetes Metab 2018;1:e00021. [Crossref] [PubMed]
- Kotlyar AM, Seifer DB. Ethnicity/Race and Age-Specific Variations of Serum AMH in Women-A Review. Front Endocrinol (Lausanne) 2020;11:593216. [Crossref] [PubMed]
- Ronn R, Bohn MK, Greenblatt EM, et al. Anti-mullerian hormone (AMH) reference values in the CALIPER cohort of healthy community children and adolescents. Clin Biochem 2022;108:63-6. [Crossref] [PubMed]
- Guibourdenche J, Lucidarme N, Chevenne D, et al. Anti-Müllerian hormone levels in serum from human foetuses and children: pattern and clinical interest. Mol Cell Endocrinol 2003;211:55-63. [Crossref] [PubMed]
- Lee MM, Donahoe PK, Hasegawa T, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab 1996;81:571-6. [PubMed]
- Zhao L, Tuo W, Wang J, et al. Age- and sex-specific reference intervals for sex hormones in children in Wuhan: a cross-sectional study of 2,477 healthy children and adolescents. Transl Pediatr 2025;14:113-26. [Crossref] [PubMed]
- Urrutia M, Grinspon RP, Rey RA. Comparing the role of anti-Müllerian hormone as a marker of FSH action in male and female fertility. Expert Rev Endocrinol Metab 2019;14:203-14. [Crossref] [PubMed]
- Fleming R, Kelsey TW, Anderson RA, et al. Interpreting human follicular recruitment and antimüllerian hormone concentrations throughout life. Fertil Steril 2012;98:1097-102. [Crossref] [PubMed]
- Silva MSB, Giacobini P. New insights into anti-Müllerian hormone role in the hypothalamic-pituitary-gonadal axis and neuroendocrine development. Cell Mol Life Sci 2021;78:1-16. [Crossref] [PubMed]


