
Single cell sequencing technology reveals the correlation between B lymphocytes and vascular inflammatory symptoms of Kawasaki disease
Highlight box
Key findings
• Single-cell sequencing identified elevated CD19+ B cells in Kawasaki disease (KD) patients, especially in the late vasculitis stage.
• A total of 1,680 differentially expressed genes were found in B cells, with key genes like IFITM1, CD55, and FCER1G down-regulated in the late stage.
• Early vasculitis was marked by high expression of S100A8, S100A9, and S100A12, which decreased over time.
What is known and what is new?
• KD is an acute vasculitis syndrome in children, often involving coronary arteries. Diagnosis relies on clinical criteria, lacking specific biomarkers. Previous studies showed increased B cells in KD, but their dynamics and functional changes during vasculitis progression were unclear.
• This study adds insights into the temporal changes of B cells and their gene expression patterns in KD, highlighting potential diagnostic markers.
What is the implication, and what should change now?
• B cell-related markers could serve as potential diagnostic indicators for KD, aiding early diagnosis and timely intravenous immunoglobulin therapy.
• Monitoring key genes like S100A8, S100A9, and S100A12 may help assess disease severity and guide treatment.
• Further validation in larger cohorts is needed to confirm their clinical utility.
Introduction
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is an acute systemic medium-sized vascular inflammatory syndrome that commonly occurs in children under 5 years old and often involves coronary arteries. It has emerged as the leading cause of acquired heart disease in the pediatric population. Coronary artery lesion (CAL) caused by the disease mainly includes coronary artery dilatation, coronary aneurysm, coronary artery stenosis and coronary artery fistula formation in the later stage. In severe cases, myocardial infarction, ischemic cardiomyopathy and even sudden death can occur (1). At present, the diagnosis of KD by most pediatricians is based on the classic criteria, which encompass the following symptoms: a fever lasting for at least 5 days, a rash, bilateral bulbar nonexudative conjunctival injection, oral changes, non-suppurative cervical lymphadenopathy, alterations in the peripheral extremities. However, the latest clinical guidelines of the United States and Japan have no longer emphasized on fever duration for the diagnosis of KD, and the guidelines also recommend that intravenous immunoglobulin (IVIG) should be given as soon as possible once the diagnosis is made, regardless of the fever duration (1,2). Timely IVIG treatment has been shown to reduce the incidence of CAL in KD children (3). Many previous researches have shown that KD is a vasculitis response disease caused by infectious agent in a small subset of genetically predisposed children, resulting in the over-activation of the immune system (4). Nevertheless, the level of immune cell activation in KD patients during the onset of vascular inflammatory symptoms post-infection remains not fully understood. Identifying reliable biomarkers for KD diagnosis and determining the optimal timing for IVIG therapy remain to be significant challenges in this domain.
The epidemiological shifts in KD incidence are noted to be age-dependent (5). The peak incidence of KD occurs at about one year of age, when the level of maternal antibodies acquired before birth declines and the development of active immunity is not mature (1). Furthermore, B lymphocyte immune responses reach maturity in children over the age of 5 years. This suggests that the underdevelopment of antibody-mediated B lymphocyte immunity could be linked to KD’s pathogenesis. Wang et al. used single-cell RNA sequencing (scRNA-seq) to analyze peripheral blood mononuclear cells (PBMCs) from KD patients, revealing a notable increase in B lymphocytes before treatment, which returned to normal post-treatment (6). Corroborating evidence from other studies has highlighted the presence of B lymphocytes and plasma cells in biopsy samples of KD-afflicted blood vessels (7). Nonetheless, the significance of the humoral immune process, particularly the role of B lymphocytes and antibodies in KD, remains a contentious issue with inconsistent findings across studies. While numerous B lymphocyte-related genes have been implicated in KD susceptibility, these findings have not translated into equivalent progress in diagnostic biomarker development (8). The onset time of clinical symptoms of KD is not uniform, and the dynamic changes of biomarkers with the appearance of different symptoms should be paid attention to, understanding these fluctuations is crucial for predicting the inflammatory course of the disease and subsequent cardiovascular complications. In this study, we conducted an analysis focusing on the alterations within PBMCs, especially B lymphocytes and their associated gene expression with varying durations of vascular inflammation symptoms in KD. We present this article in accordance with the MDAR reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-19/rc).
Methods
Patients
One hundred and two children with KD admitted to the Department of Cardiology, Shanghai Children’s Hospital from December 2019 to December 2021 were selected as the research subjects. All KD patients met the classic KD diagnostic criteria of American Heart Association (AHA) 2017 (1), including fever of unknown etiology and the presence of at least four out of the five principal clinical features (conjunctivitis, oral changes, extremity changes, rash and cervical lymphadenopathy). Patients who meet the criteria were diagnosed with complete KD. To elucidate the associations between gene expression profiles and clinical manifestations in KD patients, this study introduced a novel approach by utilizing the duration of vascular inflammation symptoms as a criterion for patient grouping, deviating from the traditional practice of relying on fever duration. The early vasculitis group was defined as the duration of all related vascular inflammation symptoms <3 days upon the diagnosis of KD. The late vasculitis group was defined as the duration of any vascular inflammatory symptoms ≥3 days upon the diagnosis of KD. All the patient received high-dose IVIG (2 g/kg) combined with oral aspirin (30 mg/kg daily) after the diagnosis of KD. Blood samples were collected at the same time points during the course of the disease in all KD children. Pre-treatment samples were collected on the day of diagnosis and before IVIG treatment, and post-treatment samples were collected 24 hours after temperature of the KD children had stabilized after IVIG treatment. Children with KD who participated in single-cell sequencing (SCS) underwent the procedure twice, before and after IVIG therapy. Healthy controls were recruited from outpatient health check-ups, consisting of individuals younger than 6 years of age with no recent history of fever, infection, or vaccination. Children with a previous history of KD and those with autoimmune diseases were also excluded from the control group. Children with common febrile diseases admitted to Shanghai Children’s Hospital during the same period were selected as the fever control group. Children who met all the following criteria were admitted as febrile control individuals: (I) definite infections, including bacterial meningitis, bacterial pneumonia, influenza, urinary tract infection, etc. (II) Fever ≥3 days. (III) Age ≥1 month and ≤5 years old. Control group children underwent SCS only once at the time of recruitment. The experimental samples were derived from leftover blood after testing. The study was prospectively registered with the Chinese Clinical Trial Register (ChiCTR) on December 24, 2019 (ChiCTR2100044729; http://www.medresman.org.cn/pub/cn/proj/projectshow.aspx?proj=7739). Written informed consent for publication was obtained from the legal guardians of all participants prior to the enrollment of this study. The study has been reviewed and approved by the Ethics Committee of Shanghai Children’s Hospital (No. 2019R081E01). This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Single-cells preparation and sequencing
For each donor, 2 mL of venous blood was collected using ethylenediaminetetraacetic acid (EDTA)-anticoagulated tubes. The fresh blood was promptly processed to prepare PBMCs suspensions, adhering to stringent criteria: cell viability exceeding 85%, total cell count surpassing 200,000, and cell diameters ranging from 7 to 60 µm. The resulting PBMC suspensions were free from red blood cells, adhesion, and any significant debris or clumps, ensuring optimal conditions for single-cell library construction, which was initiated within 30 minutes. The 5'-end library construction method from the 10× Genomics platform was utilized for library preparation. Each sample was sequenced individually on an Illumina NovaSeq platform to produce 2×150-bp paired-end reads (6). Sequencing was performed on the Illumina NovaSeq platform in PE150 mode, with the aim of sequencing 1,000 to 10,000 cells per sample, yielding an output of 30 million bases per cell for 5' gene expression profiling. Single-cell capture and library construction were carried out using the Chromium Next GEM Single Cell V(D)J Reagent Kits v1.1 from 10× Genomics, following the manufacturer’s protocol. This involved loading a cell suspension onto a Chromium Chip to create Gel Beads-in-Emulsion (GEMs), lysing the captured cells, and barcoding their transcripts via reverse transcription. The cDNA was then PCR-amplified, and libraries for scRNA-seq, scBCR-seq, and scTCR-seq were prepared using respective 10× Genomics kits.
Bioinformatics analysis
Differential expression analysis was performed for each cell type at the sample level according to the recommendations in the literature (9). The raw sequencing data, tagged with unique cell barcodes, were demultiplexed to assign reads to their respective samples. The demultiplexed reads were then aligned to the human GRCh38 reference genome using Cell Ranger software (version 7.2.0). The gene-barcode matrix generated was subsequently analyzed using Seurat (version 5.1.0) for in-depth investigation (10). Quality control measures were rigorously conducted across all samples with following criteria: the number of unique RNA molecules detected (“n_featureRNA”) was maintained between 200 and 2,500, serving as an indicator of sequencing saturation, cell size, and complexity; the mitochondrial gene percentage was set at less than 5%. The count matrix was log-normalized with a scale factor of 10,000, and the 2,000 most variable genes were identified for further dimensional reduction. The integrated matrix was scaled, and principal components from principal component analysis (PCA) were utilized for uniform manifold approximation and projection (UMAP). The optimal number of PCA dimensions for downstream clustering and visualization was determined using the ElbowPlot in Seurat. Cluster identification was performed using the FindClusters function in Seurat, applying the shared nearest neighbor (SNN) graph-based clustering algorithm on PCA-reduced data with dims set to 1:50 and a resolution of 0.5. UMAP was employed to visualize cells in a two-dimensional space. Cell identities were initially ascertained using SingleR (version 2.4.1) (11), which compared the transcriptome of each cell cluster against various reference datasets, including the human primary cell atlas, Blueprint/ENCODE, Database of Immune Cell Expression, Novershtern hematopoietic data, and Monaco immune data. Given inconsistencies and ambiguities in part of the automatic assignments, marker genes for each cell cluster were identified using Seurat’s FindMarkers function. Subsequently, the annotations of the cell clusters were manually curated based on these marker genes, incorporating the latest findings from single-cell research and utilizing the Annotation of Cell Types (ACT) web server (http://xteam.xbio.top/ACT/index.jsp). Then DESeq2 (v1.28.1) was used to analyze the differential expression between conditions, estimate the variance-mean dependence of the count data, and test the differential expression based on the negative binomial distribution. The clusterProfiler package (version 4.12.0) was used for over-representation analysis to assess the enrichment of specific biological functions or pathways among the differentially expressed genes (DEGs) with false discovery rate (FDR) <0.05 and log2fold change ≥1 between different groups. When the P value is less than 0.05, the gene is considered to be differentially expressed. Gene Ontology (GO) pathway database was retrieved from the “org.Hs.eg.db” package (version 3.19.1).
Flow cytometry
A multicolor flow cytometry protocol, specifically designed for pediatric use, was employed to analyze peripheral blood lymphocytes. Blood samples were processed immediately after collection by EDTA anticoagulant tubes. The major lymphocyte populations were labeled with BD multi-test IMK kit (Cat#662965, 1:2 dilution), which contained CD19+B, CD3+CD4+T, CD3+CD8+T, CD3−CD16+/CD56+NK cells. Antibodies were purchased from BD Bioscience (USA). After incubation at room temperature for 15 min in the dark, erythrocytes were lysed with 1 mL RBC lysis buffer (Cat#555899, BD Biosciences) for 10 min. Cells were then washed three times with 1 mL phosphate-buffered saline (PBS) and loaded onto BD FACSCantoII. Approximately 1×104 cells were collected by flow cytometry. Cell populations were gated using BD FACSDiva software.
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from human PBMC using TRIzol reagent (Invitrogen, USA; 15596-026). Subsequently, a cDNA Synthesis Kit (HiScript®1st Strand cDNA Synthesis Kit R212-02, HIScript®1st Strand cDNA Synthesis Kit, Vazyme, China) was employed for cDNA synthesis. Vazyme was utilized for the reverse transcription process. The target gene was amplified and quantified in AceQ Universal SYBR qPCR Master Mix (R511-02, Vazyme). The 2−ΔΔCq method was used to analyze the data, and the transcription level of the target gene was normalized to succinate dehydrogenase subunit A (SDHA). The primers used in the study were from AG Company (Accurate Biology, China) and the sequence is shown in the Table S1.
Statistical analysis
All statistical analyses were performed using R software. For SCS data, comparisons between annotated cells were conducted using one-way analysis of variance (ANOVA). The comparison of cell numbers detected by flow cytometry was analyzed using the Wilcoxon rank-sum test. Additionally, the comparison of DEGs among the three groups was also analyzed using the Wilcoxon rank-sum test. A P value of less than 0.05 was considered statistically significant.
Results
SCS RNA analysis of PBMC
All children participated in this study are shown in Figure 1. Eight KD patients participated in the SCS process. There were five males and three females with a mean age of 3.0±1.4 years (range, 1.6–5.3 years). The early vasculitis group comprised three children, while the late vasculitis group consisted of 4 children. All patients responded to IVIG therapy and did not develop CALs. Three children of similar age were selected as controls, including two healthy children and one child with common respiratory tract infection with fever. There were one boy and two girls, aged 1.1–5.3 years in the control group. Table 1 shows the details of children in each group.
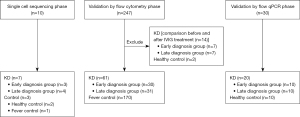
Table 1
| Characteristics | Early vasculitis group | Late vasculitis group | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | C1 | C2 | C3 | |||
| Gender | Male | Male | Male | Female | Female | Female | Male | Male | Female | Female | ||
| Age (years) | 2.1 | 2.0 | 4.7 | 1.6 | 5.3 | 3.3 | 1.9 | 1.1 | 5.3 | 4.9 | ||
| Duration of fever (days) | 4 | 6 | 4 | 3 | 5 | 6 | 6 | – | – | 4 | ||
| Congestion of the bulbar conjunctiva (days) | 1 | 1 | 2 | 3 | 5 | 2 | 5 | – | – | – | ||
| changes of lips and oral cavity (days) | 1 | 1 | 1 | 3 | 1 | 4 | 5 | – | – | – | ||
| Rash and erythema multiforme (days) | 2 | 2 | 2 | 3 | 4 | 1 | 6 | – | – | – | ||
| Swelling of the extremities (days) | 1 | 1 | 1 | 3 | – | – | – | – | – | – | ||
| Perianal flushing and desquamation (days) | – | 1 | 1 | 1 | – | 1 | 1 | – | – | – | ||
| Enlargement of cervical lymph nodes (days) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | – | – | – | ||
C3 was diagnosed with pneumonia. P, patient; C, control.
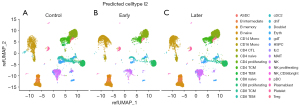
scRNA-seq was conducted on PBMCs extracted from the samples utilizing the 10× Genomics platform. There were approximately 12,000 PBMCs per sample, and 6,000 cells per sample were recovered by sequencing after loading on the platform. The total number of cells that passed quality control was 48,761, of which 34,073 were collected from KD patients before IVIG treatment and 14,688 from controls. PBMCs were pooled based on condition, revealing differences in gene expression between pretreatment KD patients, categorized by the duration of vasculitis, and the control group. The scRNA-seq data were analyzed to cluster cells from different samples, and the clustering results were depicted in a two-dimensional space as presented in Figure 2. The annotation of the cell clusters was performed using the SingleR (11). The main cell types of PBMCs were identified, including T cells, natural killer (NK) cells, B cells, monocytes, myeloid dendritic cells (mDCs), plasmacytoid dendritic cells (pDCs), hematopoietic stem/progenitor cells (HSPCS). There were also some residual red blood cells and megakaryocytes mixed in PBMCs (Figure 2, Table S2). Including CD4+T cells (28.7%), CD8+T cells (16.6%), NK cells (6.2%), B cells (26.7%), monocytes (14.5%), mDCs (<0.1%), pDCs (<0.1%) and hematopoietic stem and progenitor cells (HSPCs) (<0.1%).
Comparison of major cell subsets proportions between different groups
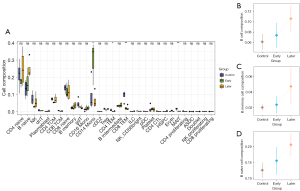
Upon analysis, it was observed that the proportion of CD19+B cells and their subsets was generally elevated in the KD group in comparison to the control group. Specifically, within the late vasculitis group, there was a notable increase in the proportion of CD19+B lymphocyte cells and B naive cells relative to the control group (P<0.05) (Figure 3A). Furthermore, although the statistical significance was not enough, the proportion of CD19+B cells and their subsets slightly increased in the late vasculitis group compared with the early vasculitis group (P=0.46) (Figure 3B-3D). T cells, including CD4+ T cells, CD8+ T cells, Treg cells, and NK cells showed a declining trend in numbers during the early stages of vasculitis. In contrast, their counts increase in the later stages compared to the early stages. Notably, the quantity of CD14 monocytes significantly rises in the early stages of vasculitis.
Validation performed by flow cytometry
To preliminarily validate our findings, we first compared the proportions of CD19+B cells in 14 KD children, including seven children who underwent SCS, before and after IVIG treatment with those of two healthy controls. This included seven cases in the early vasculitis group and 7 cases in the late vasculitis group. The proportion of CD19+B cells increased obviously before IVIG treatment and decreased to normal level after IVIG treatment (P=0.006) in KD patients, which was consistent with the results of single cell sequencing. Consistent with the findings of SCS results, the proportion of CD19+B lymphocytes in late vasculitis group. was also higher than that in early vasculitis group, but there was no significant difference (P=0.06) (Figure 4A). In order to verify whether the increase of CD19+B lymphocytes in the acute phase of KD is related to fever duration, we also compared the flow cytometry data of 61 children with KD before IVIG treatment including 30 cases in the early vasculitis group and 31 cases in the late vasculitis group and 170 children with fever who were admitted to the hospital during the same period. The data showed that the proportion of CD19+B lymphocytes in the acute phase of KD was significantly higher than that in the febrile control group (P=0.008), but there was no significant difference in the absolute value of CD19+B lymphocytes in early vasculitis group compared with that in the febrile control group. The proportion (P=2.8E−07) and absolute value (P=0.009) of CD19+B cells in late vasculitis group were also higher than those in early vasculitis group (P=2.8E−07) (Figure 4B). Comparing the relationship between fever duration days and CD19+B lymphocytes on the day of sampling in 61 children with KD, it was found that the percentage and absolute value of CD19+B lymphocytes did not have a detailed trend of change with fever duration days (Figure 4C).

Functional enrichment analysis of DEGs in B lymphocytes
To identify the genes involved in the progression of KD vasculitis, DESeq2 software was used to analyze the differences in the expression of CD19+B lymphocytes cells between the early and late vasculitis group. A total of 1,680 DEGs were identified (Table S3, Figure 5). GO and pathway over-representation analyses of the DEGs suggested that they were mainly involved in two domains. The cellular processes under investigation were multifaceted, encompassing the activation of B cells, the B cell-mediated immune response, and the immunoglobulin-mediated immune response. Concurrently, these processes were intricately linked to viral entry into host cells, the directed migration of granulocytes, the processing and presentation of exogenous antigens, and the adaptive immune response. These complex immunological interactions potentially shed light on the etiological mechanisms underlying KD.
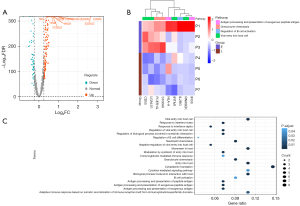
The core genes involved in these pathways included IFITM1, CD55, FCER1G, HLA-A, S100A8, S100A9, S100A12, RNASEK, IFI30, LGALS1, and IL2RG. The expression level of these above genes in late vasculitis group was down-regulated compared with that in early vasculitis group (Figure 5B). This also indicates that with the prolonged duration of KD vasculitis, although the number of B cells increases, the function of these key genes is down-regulated and B cell-related functions are reduced.
Temporal expression analysis of DEGs in B lymphocytes
We performed a time-series analysis of CD19+B lymphocytes and genes separately with the time of onset of vasculitic symptoms (Figure 6). Genes with similar expression patterns were divided into clusters by the Mfuzz package, and eight clusters were finally obtained. The time points were defined as normal, the time of onset of vasculitic symptoms less than three days, and the time of onset of vasculitic symptoms ≥3 days. The group with the most gene enrichment is cluster 2. It can be seen that some genes have high expression in the early stage of vasculitis, and with the extension of the duration of vasculitis, the expression of these genes is down-regulated, including S100A8, S100A9, and S100A12 genes (Table S4).
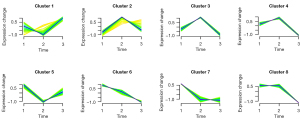
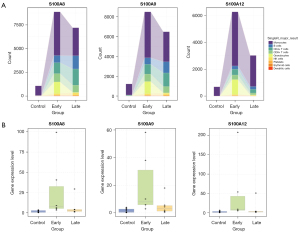
S100A8, S100A9, S100A12 increased significantly in the early stage of vascular inflammation in KD
Initially, a comparative analysis of transcriptome levels for S100A8, S100A9, and S100A12 was conducted across PBMCs from all subjects. The SCS data revealed significant upregulation of these genes in diverse cellular populations within the early vasculitis group (Figure 7A). Furthermore, we utilized qPCR method as validation to detect 20 KD children, including 10 patients belongs to early vasculitis group and 10 patients belongs to late vasculitis group, and 10 patients belongs to healthy control group. According to the qPCR results, S100A8, S100A9 and S100A12 were also higher than other stage in the early stage of vasculitis (P<0.05) (Figure 7B).
Discussion
Utilizing SCS technology to delve into the cellular and molecular intricacies of the human immune system offers a powerful means to uncover the nuanced mechanisms underlying immune responses. This approach can shed light on the emergence and progression of immune-mediated diseases, thereby enhancing our understanding of pathogenesis, informing clinical practices, and guiding drug development. Given the profound insights SCS can provide into immune system dynamics, its application to analyze immune cell alterations during the acute phase of KD holds promise for uncovering novel immune cell types, immune pathways, and specific immune markers. Such discoveries could significantly advance our knowledge of KD’s etiology (12). After constructing an immunological map of KD, it was revealed that, compared to healthy controls, the KD group showed a notable increase in the proportion of B lymphocytes before treatment, which normalized after treatment (6). Recently, Fan et al. (13) have also focused on the shifts in various immune cell subsets in KD, both pre- and post-IVIG therapy, and have examined the biological processes associated with differential gene expression in KD and healthy controls throughout the disease course. A previous study involving analysis of two KD samples identified 12 distinct cell populations. It was observed that children with KD have a reduction in naive CD8+T lymphocytes, T helper cells, and B lymphocytes, with a primary focus on immune-related T lymphocytes and natural killer T (NKT) lymphocytes (13). Notably, the observed trends related to B lymphocytes diverge from our analysis, suggesting that sample size and disease progression are both critical factors to consider. Chang et al. reported a case study of a 4-year-old boy with KD, where PBMCs and B lymphocytes were isolated from the obtained blood samples drawn both prior to and following IVIG therapy. The plasma cell response in this KD patient was found to mirror those seen in children with other infections. However, the plasma cells in this patient showed a significant amplification of antibodies against the IGHV4-34 gene, which has been linked to autoimmunity in previous research (14). In terms of cell trajectory analysis, it has been reported that a subpopulation of monocytes with high SELL gene expression in KD infants, characterized by poor differentiation, is implicated in neutrophil activation. Additionally, the study found that the three monocyte subsets represented a linear differentiation trajectory, each with distinct biological functions, with classical monocytes exhibiting more pro-inflammatory phenotypes (15).
The objective of our study is to investigate the changes in the number and function of B lymphocytes and their subsets in relation to the duration of KD vasculitis. By doing so, we aim to provide further insights into the immunopathogenesis of KD and identify potential therapeutic targets. Genetic research has demonstrated that susceptibility genes for KD participate in the development and function of B cells (16-18), underscoring the critical role of B cell immunity in KD. The dysfunction in B cell development may be the underlying mechanism for the delayed immune maturation observed in KD (16). However, the potential pathogenic mechanisms have not yet been elucidated. Our findings indicate that the proportion of B lymphocytes not only increases in KD children but also correlates with the duration of vascular inflammation. Currently, research is not only focused on the differences between B lymphocytes in the acute phase of KD and those in common febrile illnesses but also extends to the plasmablast cells that are overproduced in the phase following B lymphocyte stimulation by inflammation. This suggests that KD symptoms manifest post-infection and vaccination. However, when compared to common infectious diseases, there is no significant difference in plasmablast cells during the acute phase of KD. It is crucial to consider whether the degree of vascular inflammation in the child at the time of sampling influences these findings (17,19). This is of great significance for the study of KD pathogenesis and the identification of biomarkers. The proportion of B lymphocytes in the acute phase of KD has shown variation in past studies, which we believe is related to the timing of sampling. Previous study has indicated that a low expression level of T lymphocytes coupled with a high expression level of B lymphocytes may be associated with complete KD, whereas atypical KD does not show as significant a difference as complete KD, which could be related to the severity of KD vasculitis (20). This is in line with our hypothesis that the percentage of B lymphocytes may play a role in the differential diagnosis and assessment of KD severity. For peripheral blood immune cells in the acute phase of KD, it has been observed that while the number and function of adaptive immune cells in peripheral blood are still a subject of debate, the proportion of B lymphocytes in the coronary tissue of KD children remains increased even after IVIG treatment. This suggests that B lymphocytes can selectively transfer from peripheral blood to vascular tissue (21). Obviously, further research on the diagnostic scoring system that combines clinical symptoms and fluctuations in immune cell counts and inflammatory markers. Other studies have shown that although the quantity of B cells increases in the acute phase of KD, there is a decrease in IgG levels and B cell diversity. This suggests that most B lymphocytes in the acute phase of KD may be non-functional, immature, or not activated (16,22).
In our study, we conducted a functional analysis of DEGs in B lymphocytes and discovered their involvement in key immunological processes, such as B cell activation, B cell-mediated immune response, and immunoglobulin-mediated immune response. This finding might suggest a hypothesis that in KD, while the count of B cells increases with the chronicity of the disease, their functionality may be compromised. This is also confirmed in the co-expression network results. With the extension of the duration of vascular inflammation, the specialized functional modules of B cells gradually decreased. Notably, genes such as FCER1G, CD55, IL2RG, and HLA were observed to be down-regulated as the duration of vascular inflammation in KD prolonged. Fc receptors encoded by FCER1G play a pivotal role in immune regulation, recognizing immunoglobulin E (IgE) and modulating immune responses (23). Study on KD have indicated that FCER1G mRNA expression is elevated in KD children compared with healthy controls, suggesting a critical role of FCER1G in KD pathophysiology (24). However, no significant difference was found in the expression of FCER1G between KD patients and the fever control group (25). The CD55 gene, which encodes a membrane-bound complement regulatory protein, is significant in the treatment of B-cell associated lymphoma with rituximab, CD55 plays a crucial role in regulating immune responses and inflammation by inhibiting the over-activation of the complement system, which helps to prevent autoimmune reactions and inflammatory damage (26). IL2RG is also implicated in the proliferation and activation of B lymphocytes (27,28). The precise role of these genes in KD remains to be elucidated. Furthermore, DEGs were associated with a range of functions, including virus entry into host cells, granulocyte chemotaxis, antigen processing and presentation of exogenous antigens, adaptive immune responses, and cytokine signaling pathways. Genes such as IFITM, S100, RNASEK, and LGACS1 were primarily involved. Our previous findings indicated higher expression of the S100 gene across lymphocyte populations in KD patients compared to healthy controls (6). The S100 series proteins are known for their proinflammatory roles in KD, including neutrophil release and chemotaxis to the vascular endothelium (29,30). However, our results show a down-regulation of S100A9 expression in B cells with the prolonged duration of vasculitis, indicating a complex role that requires further investigation. IFITM and RNASEK are associated with viral entry into the host and the activation of subsequent immune responses (31,32). KD shares vascular inflammatory symptoms with other inflammatory response syndromes, such as coronavirus disease 2019 (COVID-19), suggesting a possible viral etiology for KD, although this remains unconfirmed (33). In the treatment of KD children unresponsive to IVIG, treatments involving extensive immunosuppressants like corticosteroids and tumor necrosis factor-α (TNF-α) monoclonal antibodies have been used, indicating that T lymphocyte regulation may play a more significant role in this group of patients (34). The interplay between the pathways involved in the functions of the differential genes, along with both humoral and cellular immunity, in KD pathogenesis merits further study. Beyond the essential role of B lymphocytes in humoral immunity, studies have shown that antibody memory cells, such as IgG + ASCS, are significantly increased in the acute phase of KD. These cells promote B cell proliferation and are significantly correlated with C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) levels, indicating their importance in KD’s immunopathogenesis (35).
A co-expression network analysis was performed on B cells, revealing associations between the functional diversification of these cells and the chronicity of vasculitis symptoms. We found that a large number of genes were specifically increased in early vasculitis, in which S100A8, S100A9, S100A12 were particularly obvious. This aroused our interest, and we simultaneously analyzed the trends of these three genes in all transcriptomes and obtained the same results, which were verified by multi-copy qPCR. The complexes formed by S100A8, S100A9 and S100A12 play a key role in inflammation and immune response, and they regulate cell signaling and affect cell behavior and tissue function by binding to different cell surface receptors. The specific role of these complexes may vary depending on cell type, microenvironment, and pathological conditions. S100A8, S100A9, and S100A12 not only form complexes, but can also interact with other proteins and cellular structures to influence a variety of processes both inside and outside the cell (36,37). Specifically, S100A8 and S100A9 can combine to form the heterodimer calprotectin, which binds calcium ions and modulates immune cell migration, activation, and signaling in inflammatory responses (36). Under inflammatory conditions, activated myeloid cells such as monocytes and neutrophils release S100A8/A9 heterodimers. S100A8 and S100A9 act as damage-associated molecular patterns (DAMPs) molecules and regulate inflammatory processes by binding to a variety of cell surface receptors such as Toll-like receptor 4 (TLR4) and advanced glycation end product receptor (RAGE) (38). Their increased expression levels in inflammatory and autoimmune states suggest that they play an important role in both inflammatory responses and cardiovascular disease (39,40). S100A8 and S100A9 can chemotaxis to endothelial cells and activate endothelial cells through binding with RAGE on endothelial cells, resulting in increased production of cytokines and chemokines, thus promoting recruitment of inflammatory cells and amplification of inflammatory response, and participating in the occurrence of systemic vasculitis. At the same time, S100A8/A9 can promote the increase of NLRP3/GSDMD-mediated macrophage pyroptosis induced release of inflammatory factors, thereby amplifying the abnormal immune response and forming a positive feedback system (41-43). Researchers also found that serum levels of S100A8/A9 heterodimer were significantly elevated in KD patients and correlated with disease activity. Future studies may focus on the process of molecular chemotaxis and activation of endothelial cells in these three injury-related molecular modes. It is possible that this effect may lead to inflammation and damage of coronary arteries, possibly increasing the risk of endothelial scorch death (30,44). For the phenomenon that the quantities of CD14+ monocytes and S100A8, S100A9, S100A12 are significantly increased in the early stage of vascular inflammation, and the CD19+B lymphocytes continue to increase with vascular inflammation, they may serve as biomarkers of disease activity and severity. Changes in their expression levels may help us monitor the progression of the disease and the effects of treatment.
Conclusions
In conclusion, our study establishes the connection between the timing of vascular inflammation and the pathogenesis of KD. We have discovered a significant association between the duration of vascular inflammation and the immunological markers that characterize KD. Specifically, the proportion and quantity of CD19+B lymphocytes, alongside the expression levels of S100A8, S100A9, and S100A12, are intricately linked to how long vascular inflammation persists in KD. The intricate relationship between immune cell dynamics and the temporal profile of vascular inflammation presents a compelling avenue for future research and therapeutic development in KD.
Acknowledgments
We wish to thank Professor Zhen Wang from Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences for his suggestions on this article.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-19/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-19/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-19/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-19/coif). J.H., Y.X. and G.L. are employed by the company Daozhi Precision Medicine Technology (Shanghai) Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study has been reviewed and approved by the Ethics Committee of Shanghai Children’s Hospital (No. 2019R081E01). Written informed consent for publication was obtained from the legal guardians of all participants prior to the enrollment of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017;135:e927-99. [Crossref] [PubMed]
- Kobayashi T, Ayusawa M, Suzuki H, et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr Int 2020;62:1135-8.
- Makino N, Nakamura Y, Yashiro M, et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015-2016. Pediatr Int 2019;61:397-403. [Crossref] [PubMed]
- Kuo HC, Pan CT, Huang YH, et al. Global Investigation of Immune Repertoire Suggests Kawasaki Disease Has Infectious Cause. Circ J 2019;83:2070-8. [Crossref] [PubMed]
- Nakamura Y. Kawasaki disease: epidemiology and the lessons from it. Int J Rheum Dis 2018;21:16-9. [Crossref] [PubMed]
- Wang Z, Xie L, Ding G, et al. Single-cell RNA sequencing of peripheral blood mononuclear cells from acute Kawasaki disease patients. Nat Commun 2021;12:5444. [Crossref] [PubMed]
- Takahashi K, Oharaseki T, Naoe S, et al. Neutrophilic involvement in the damage to coronary arteries in acute stage of Kawasaki disease. Pediatr Int 2005;47:305-10. [Crossref] [PubMed]
- Kwon YC, Kim JJ, Yun SW, et al. BCL2L11 Is Associated With Kawasaki Disease in Intravenous Immunoglobulin Responder Patients. Circ Genom Precis Med 2018;11:e002020. [Crossref] [PubMed]
- Amezquita RA, Lun ATL, Becht E, et al. Orchestrating single-cell analysis with Bioconductor. Nat Methods 2020;17:137-45. [Crossref] [PubMed]
- Hao Y, Stuart T, Kowalski MH, et al. Dictionary learning for integrative, multimodal and scalable single-cell analysis. Nat Biotechnol 2024;42:293-304. [Crossref] [PubMed]
- Aran D, Looney AP, Liu L, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol 2019;20:163-72. [Crossref] [PubMed]
- Stoeckius M, Hafemeister C, Stephenson W, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods 2017;14:865-8. [Crossref] [PubMed]
- Fan X, Zhou Y, Guo X, et al. Utilizing single-cell RNA sequencing for analyzing the characteristics of PBMC in patients with Kawasaki disease. BMC Pediatr 2021;21:277. [Crossref] [PubMed]
- Chang AJ, Baron S, Hoffman J, et al. Clonal expansion and markers of directed mutation of IGHV4-34 B cells in plasmablasts during Kawasaki disease. Mol Immunol 2022;145:67-77. [Crossref] [PubMed]
- Geng Z, Tao Y, Zheng F, et al. Altered Monocyte Subsets in Kawasaki Disease Revealed by Single-cell RNA-Sequencing. J Inflamm Res 2021;14:885-96. [Crossref] [PubMed]
- Lee JK. Hygiene Hypothesis as the Etiology of Kawasaki Disease: Dysregulation of Early B Cell Development. Int J Mol Sci 2021;22:12334. [Crossref] [PubMed]
- Lindquist ME, Hicar MD. B Cells and Antibodies in Kawasaki Disease. Int J Mol Sci 2019;20:1834. [Crossref] [PubMed]
- Lee YC, Kuo HC, Chang JS, et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet 2012;44:522-5. [Crossref] [PubMed]
- Martin M, Wrotniak BH, Hicar M. Suppressed plasmablast responses in febrile infants, including children with Kawasaki disease. PLoS One 2018;13:e0193539. [Crossref] [PubMed]
- Ding Y, Li G, Xiong LJ, et al. Profiles of responses of immunological factors to different subtypes of Kawasaki disease. BMC Musculoskelet Disord 2015;16:315. [Crossref] [PubMed]
- Xie Z, Huang Y, Li X, et al. Atlas of circulating immune cells in Kawasaki disease. Int Immunopharmacol 2022;102:108396. [Crossref] [PubMed]
- Ko TM, Kiyotani K, Chang JS, et al. Immunoglobulin profiling identifies unique signatures in patients with Kawasaki disease during intravenous immunoglobulin treatment. Hum Mol Genet 2018;27:2671-7. [Crossref] [PubMed]
- Srivastava P, Bamba C, Pilania RK, et al. Exploration of Potential Biomarker Genes and Pathways in Kawasaki Disease: An Integrated in-Silico Approach. Front Genet 2022;13:849834. [Crossref] [PubMed]
- Khor CC, Davila S, Breunis WB, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet 2011;43:1241-6. [Crossref] [PubMed]
- Chang LS, Ming-Huey Guo M, Lo MH, et al. Identification of increased expression of activating Fc receptors and novel findings regarding distinct IgE and IgM receptors in Kawasaki disease. Pediatr Res 2021;89:191-7. [Crossref] [PubMed]
- Salles G, Barrett M, Foà R, et al. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv Ther 2017;34:2232-73. [Crossref] [PubMed]
- Aliyari Z, Soleimanirad S, Sayyah Melli M, et al. IL2rg Cytokines Enhance Umbilical Cord Blood CD34+ Cells Differentiation to T Cells. Adv Pharm Bull 2015;5:615-9. [Crossref] [PubMed]
- Meazza R, Azzarone B, Orengo AM, et al. Role of common-gamma chain cytokines in NK cell development and function: perspectives for immunotherapy. J Biomed Biotechnol 2011;2011:861920. [PubMed]
- Perera C, McNeil HP, Geczy CL. S100 Calgranulins in inflammatory arthritis. Immunol Cell Biol 2010;88:41-9. [Crossref] [PubMed]
- Tacke CE, Burgner D, Kuipers IM, et al. Management of acute and refractory Kawasaki disease. Expert Rev Anti Infect Ther 2012;10:1203-15. [Crossref] [PubMed]
- Gómez-Herranz M, Taylor J, Sloan RD. IFITM proteins: Understanding their diverse roles in viral infection, cancer, and immunity. J Biol Chem 2023;299:102741. [Crossref] [PubMed]
- Hackett BA, Yasunaga A, Panda D, et al. RNASEK is required for internalization of diverse acid-dependent viruses. Proc Natl Acad Sci U S A 2015;112:7797-802. [Crossref] [PubMed]
- Rowley AH, Shulman ST, Arditi M. Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J Clin Invest 2020;130:5619-21. [Crossref] [PubMed]
- Burns JC, Koné-Paut I, Kuijpers T, et al. Review: Found in Translation: International Initiatives Pursuing Interleukin-1 Blockade for Treatment of Acute Kawasaki Disease. Arthritis Rheumatol 2017;69:268-76. [Crossref] [PubMed]
- Xu M, Jiang Y, Wang J, et al. Distinct variations of antibody secreting cells and memory B cells during the course of Kawasaki disease. BMC Immunol 2019;20:16. [Crossref] [PubMed]
- Pruenster M, Vogl T, Roth J, et al. S100A8/A9: From basic science to clinical application. Pharmacol Ther 2016;167:120-31. [Crossref] [PubMed]
- Pietzsch J, Hoppmann S. Human S100A12: a novel key player in inflammation? Amino Acids 2009;36:381-9. [Crossref] [PubMed]
- Wang S, Song R, Wang Z, et al. S100A8/A9 in Inflammation. Front Immunol 2018;9:1298. [Crossref] [PubMed]
- Averill MM, Kerkhoff C, Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol 2012;32:223-9. [Crossref] [PubMed]
- Cai Z, Xie Q, Hu T, et al. S100A8/A9 in Myocardial Infarction: A Promising Biomarker and Therapeutic Target. Front Cell Dev Biol 2020;8:603902. [Crossref] [PubMed]
- Tan X, Zheng X, Huang Z, et al. Involvement of S100A8/A9-TLR4-NLRP3 Inflammasome Pathway in Contrast-Induced Acute Kidney Injury. Cell Physiol Biochem 2017;43:209-22. [Crossref] [PubMed]
- Fang X, Lian H, Liu S, et al. A positive feedback cycle between the alarmin S100A8/A9 and NLRP3 inflammasome-GSDMD signalling reinforces the innate immune response in Candida albicans keratitis. Inflamm Res 2023;72:1485-500. [Crossref] [PubMed]
- Jorch SK, McNally A, Berger P, et al. Complex regulation of alarmins S100A8/A9 and secretion via gasdermin D pores exacerbates autoinflammation in familial Mediterranean fever. J Allergy Clin Immunol 2023;152:230-43. [Crossref] [PubMed]
- Beltran JVB, Lin FP, Chang CL, et al. Single-Cell Meta-Analysis of Neutrophil Activation in Kawasaki Disease and Multisystem Inflammatory Syndrome in Children Reveals Potential Shared Immunological Drivers. Circulation 2023;148:1778-96. [Crossref] [PubMed]


