
Hypoplastic left heart syndrome—a scoping review
Highlight box
Key findings
• Hypoplastic left heart syndrome (HLHS) remains genetically heterogeneous.
What is known and what is new?
• It is known that HLHS is one of the most complex congenital heart defects, characterized by a small or hypoplastic left ventricle, small or hypoplastic left-sided heart structures, and a dominant right ventricle.
• The scoping review confirms that there is evidence of incomplete penetrance for the C57Bl/6J-b2b635Clo/J (Ohia) mice. Two of the five homozygous mutations found in the Ohia line with two founder HLHS mutants are necessary for the disease.
What is the implication, and what should change now?
• Progress in research has been impeded by the absence of genetically modified animal models that accurately replicate human disease, although the Ohia mice seem to currently be the best animal model so far. These findings may critically support a new paradigm for the complicated genetics of this congenital heart defect and there is some evidence that HLHS can indeed originate genetically in a combinatorial approach.
Introduction
In the absence of surgical intervention, hypoplastic left heart syndrome (HLHS) is a consistently lethal condition. It manifests in around 3% of all neonates born with congenital heart illness. Dr. Bardeleben, a German pathologist, published the initial report detailing such a patient in 1851 (1,2). The initial description of the constellation of abnormalities comprising the condition was provided by Lev, who focused on the hypoplasia of the aortic tract (3). Noonan and Nadas consolidated the anatomical observations to establish a distinct syndrome (4). A recently unearthed infant mummy in South America (Peru), dating back 6,500 years, was determined to possess a similar heart morphologic configuration (5). The condition affects approximately 1,000 to 2,000 newborns annually in the United States. This scoping review was assiduously carried out to identify, synthesize, and iwis analyze the scientific knowledge on the etiology and pathogenesis of the HLHS.
Methods
The author used a scoping review approach to carry out this comprehensive review. Following the advice of the Joanna Briggs Institute, I usually conduct the scoping reviews (6). The author is Master Public Health graduated other than MD and PhD and has solidified this approach in several published papers. The following stages served as the basis for organizing the review: the following steps were taken to complete the review: establishing a guiding question and objective, creating a search strategy, searching databases, selecting articles based on titles and abstracts, selecting scientific articles based on full text reading, summarizing the findings, and presenting and discussing the results in a descriptive style. The objective was to identify, synthesize, and analyze the scientific knowledge on HLHS in a scoping review thoroughly. The search for articles was conducted between January 1, 2019 and February 20, 2025 (articles were published between January 1st, 2019 to August 31st, 2024) on the PubMed/MEDLINE, Scopus, Web of Science, and Cochrane databases. This search was complemented by a gray search including internet browsers (e.g., Google) and textbooks on the HLHS. The following research question guided the study: “What are the basic data on the etiology and pathogenesis on HLHS?” All stages of the selection process were comprehensively carried out by the single author. The inclusion criteria were articles that contained the three PCC (population, concept, and context) elements. The articles should answer the research question. They were written in English (language) in the selected period. Articles written in other languages and that did not answer the guiding question were excluded. For the specific search, well-delineated descriptors in health sciences and Medical Subject Headings (MeSH) were also used for each item of the strategy and its related terms. To combine descriptors, the AND, OR, and NOT Boolean operators were used. As indicated, this paper is a comprehensive scoping review following the PRISMA-ScR guidelines to make the statements and arguments of this review strong and verifiable (7,8) (available at https://tp.amegroups.com/article/view/10.21037/tp-24-367/rc).
Results
Of the 1,364 articles found, 75 were included in the sample for analysis, which was implemented with an additional 25 articles from references and gray literature (e.g., medical textbooks, websites). The studies analyzed allowed to delineate the HLHS, particularly the phenotype, but also the etiology and illustrate where new investigations may be needed.
Phenotype
Technically, the syndrome is a congenital disorder of fetal development, as the ventricular septum is inherently undamaged (2,9). Accurate identification of the specific phenotypic characteristics of individuals with the syndrome is crucial, as left ventricular hypoplasia can be observed in various other conditions. These patients encompass those with an imbalanced atrioventricular canal defect, a right ventricle with two outlets, discordant (conflicting) ventriculo-arterial cardio-vasal connections, or a common arterial trunk obstruction. These examples may involve a deficiency of the ventricular septum. Therefore, these are separate disease entities regarding characteristic features and underlying mechanisms.
Furthermore, there is additional variation in phenotypes among the cohort of pediatric and adult patients accurately identified as having HLHS. This is contingent upon the heterogeneity in the structure of the mitral and aortic cardiac valves, the extent of underdevelopment of the left ventricle, and the dimensions of the aorta. Unsurprisingly, the wide range of phenotypic variations has impeded research efforts to clarify the molecular processes responsible for the syndrome and may potentially impact a specific treatment of the condition.
Furthermore, individuals with the syndrome often exhibit abnormalities in the left-sided atrial chambers and the extra-pericardial aortic pathways. The most prevalent form of the syndrome is characterized by a significant thickening of the left ventricular wall, which reduces the size of the left ventricular cavity of the heart. This reduction is insufficient to support the whole cardiac output of the body (Figure 1).
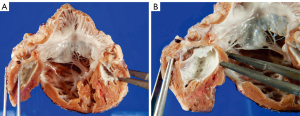
This genetic variation is linked to the narrowing of the mitral valve and often to the development of aortic atresia. An inherent characteristic of the hypoplastic left ventricle in this particular form is the presence of a substantial layer of fibroelastosis that coats its inner cavity, as demonstrated in Figure 1. In the context of aortic atresia, the hypoplastic ascending aorta functions merely as a channel that supplies blood to the coronary arteries in a backward flow. In some instances, however, the aortic valve may exhibit severe constriction rather than atresia yet nonetheless possess a thus defined thick-walled and underdeveloped left ventricle, which too possesses a remarkable fibro-elastotic lining. The second most prevalent phenotypic type is characterized by an atretic rather than stenotic mitral valve. Invariably, this change is linked to aortic atresia. As a result of mitral atresia, blood is unable to access the left ventricle. Consequently, the ventricular cavity may be slit-like, very small, or even practically nonexistent to the naked eye (Figure 2). The thickness of its walls is significantly reduced compared to when the mitral valve is stenotic. The fibro-elastotic layer typical of mitral stenosis is absent in the endocardial lining of the left ventricle. Furthermore, there exists a third variation, which is the least common. This particular variant has diminutive mitral and aortic valves, which are consistent with the dimensions of the hypoplastic left ventricle. The variation above has been characterized as the “hypoplastic left heart complex” (10). The entity may be described as a somewhat severe aortic coarctation with concomitant left ventricular hypoplasia since the most prominent characteristic of the pattern is the “blocked” aortic arch. The predominant group of hearts first documented by Noonan and Lev exhibited this characteristic. Instances of this individual variation, however, can be located in historical records where they have been categorized as indicative of “hypoplastic left heart syndrome” by the diagnosing pathologists (9). Indeed, it is noteworthy that, up to now, the mechanisms responsible for the emergence of any of the various variations have not been identified.

Theories of aberrant morphogenesis
Two divergent hypotheses have been suggested to elucidate the etiology. The first one, which suggests anomalous flow, proposes that the smaller left ventricular cardiac cavity is due to diminished flow across the mitral valve, resulting in reduced specific growth of the structures on the left heart side. Experimental evidence has demonstrated that compromised anterograde flow to the developing ventricle in animal models leads to altered ventricular development, supporting this hypothesis. The models above have shown a reduced increase in left ventricular cardiomyocytes (11). However, in most situations, relieving a blocked aortic valve during embryonic and fetal life in humans does not effectively avoid the further progression of the disease (12). Endocardial fibroelastosis, in conjunction with mitral stenosis, is observed in most fetuses who develop the symptoms of the illness following this surgery. This marker indicates endocardial damage, implying that other processes may contribute to the hypoplasia of the left ventricle. However, it is also plausible that any capacity to react to the reinstatement of regular hemodynamics has already been gone by the time of intervention. At the E12.5 stage, mouse endocardial cells undergo resorption of primary cilia necessary for detecting fluid shear stress. Aberrations in multiple genes associated with ciliary function, including Ift88, Kif3a, Lrd, and Pkd2 lead to impaired maturing process of the developing left ventricle (13). However, the specific developmental stage at which this event occurs in the human fetus remains unknown.
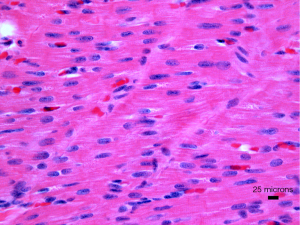
The second hypothesis is that the significant mechanism is the hypoplasia of the left ventricle. Specifically, it is well-established that certain patients exhibit substantial left ventricular hypoplasia even when there is no significant mitral or aortic stenosis present. There have been documented cases of mutations in a specific gene, notably NKX2-5, linked to left cardiac hypoplasia and cardiomyopathy (14,15). Furthermore, there have been accounts of individuals with the syndrome displaying a phenomenon known as “non-compaction” of the right ventricle. Indeed, this condition denotes an overoccurrence of right ventricular trabeculation. These data offer additional validation to propose that hypoplastic left heart syndrome could represent another form of primary cardiomyopathy in newborns (16). Cellularly, tissue samples from the explanted hearts of babies have shown an elevated fibrosis level accompanied by disorganization and hypertrophy of the cardiomyocytes (Figure 3) (17). The precise anatomical combinations in the examined cases remain to be detailed (17). Previously, cardiac tissue of the left ventricle was collected from the hearts of fetuses diagnosed with the condition between 20 and 27 weeks gestational age (18). The authors documented a decline in cardiac progenitors and a drop in cardiomyocytes and endocardial cells. Their findings also revealed elevated quantities of smooth muscular cells and myofibroblasts. Analysis of the tissues at the molecular level revealed reduced formation of blood vessels (hypo-angiogenesis), characterized by increased expression of hypoxia-inducible factor 1 alpha (HIF-α), cellular senescence linked to cancer genes, and fibrosis-related with TGF-beta protein (TGF-β). These investigations are essential because left ventricular fibrosis has been identified as a crucial element of the condition, especially in mitral stenosis (9). The critical question is whether the results are secondary, caused by abnormal hemodynamics resulting in reduced blood flow, or if they are part of the first and leading illness process that highlights the cardiac ventricular hypoplasia. It is feasible to elucidate the fundamental cellular and molecular processes contributing to the formation of hypoplastic left ventricular cavity and mural thickening in the early phases of heart development. The most effective approach is employing genetically modified animal models as long as these models faithfully represent the phenotypic diversity observed in the clinical environment.
Animal models
Utilizing genetically modified animal models to study human genes involved in a genetic syndrome is intriguing. Despite convincing proof suggesting a genetic origin for the illness, only a limited number of perhaps disease-causing genes in humans have been discovered. To our knowledge, the genes that have been discovered hitherto include NKX2-5 (14), NOTCH1 (19), ETS1 (20,21), HAND1 (22), and rbFOX2 (23). However, mutations in these five genes likely represent, at most, a narrow subgroup, possibly 1/10, of all individuals born with HLHS (24). In the majority of instances, thus, the precise genetic cause remains unidentified and worth further investigation. These observations would indicate a complex and multifaceted cause for this cardiologic disease.
Furthermore, the “Baltimore-Washington Infant Study Group” has established that environmental factors are linked to a higher occurrence of the syndrome (25). Such environmental factors may include but are not limited to parental exposure to organic solvents. More recently, epigenetic variables, particularly those specific to NKX2-5, have been implicated, indicating that the syndrome may result from genetic, epigenetic, and environmental influences (26). This knowledge may help expose the challenge of developing suitable genetically modified animal models for the condition. Accurate demonstration of the suitable phenotypic anatomy in such animal models would offer a unique chance to acquire unprecedented insights into the early molecular and cellular processes that occur during the developmental alterations leading to the condition. To date, no accurate models have been developed demonstrating left ventricular hypoplasia in the presence of a complete ventricular septum and associated atrio-ventricular (cardio-cardial) and ventriculo-arterial (cardio-vasal) connections, apart probably from the Ohia mouse model. Fetal hypoxia exposure has been verified in some models, one of which identified left ventricular dysfunction clearly (27).
In an animal model studied in our laboratories (Kumar et al. 2020) (27), the aim was to investigate the potential correlation between fetal hypoxia exposure, a consequence of placental insufficiency, and the development of left ventricular dysfunction. My research group and I identified aortic stiffness during the early stages of postnatal development. The pregnant Sprague Dawley rats were subjected to hypoxia events (11.5% FiO2-fraction of inspired oxygen) starting from embryonic day E15–21, or they were treated with normoxic treatments (controls). Echocardiography was used to longitudinally evaluate left ventricular cardiac function and aortic pulse wave velocity (a marker of “aortic stiffness”) from day 1 to week 8 after birth. Myocyte nuclear morphology and collagen fiber properties were assessed using myocardial hematoxylin and eosin and picro-sirius staining, respectively. Transient increases in systolic function parameters were observed after exposure to hypoxia, mainly at week 2 (P<0.008) and diastolic dysfunction advanced after an exposure to fetal hypoxia starting from weeks 1–2, characterized by reduced early inflow Doppler velocities, as well as a smaller rise in early to late “inflow velocity ratios” and, remarkably, “annular and septal E’/A’ tissue velocities” compared to the control group (P<0.008). Further support of modified diastolic function was observed by the considerably reduced isovolumetric relaxation time compared to the cardiac cycle after exposure to hypoxia starting from week 1 (P<0.008). The status of “stiffness of the aorta” increased after hypoxia from day 1 to week 8 (P<0.008, except week 4). In addition, exposure to hypoxia was specifically linked to changes in nuclear morphology at week 2 and an increase in collagen fiber thickness at week 4. It seems clear that the model of chronic fetal hypoxia in Kumar et al. (27) is related to the gradual deterioration of left ventricular diastolic performance, which is accompanied by alterations in nuclear morphology and collagen fiber thickness, as well as an increase in aortic stiffness starting from early postnatal periods.
Prior research has identified the ETS1 gene as a potential factor contributing to left ventricular heart hypoplasia in individuals with Jacobsen syndrome (20). Within this genetic disease, approximately 20% of all newborns exhibit the identifiable phenotypic characteristics of HLHS. Furthermore, a novel mutation in the ETS1 gene has been detected in a patient with a hypoplastic left ventricle variation, in addition to various other clinical characteristics of Jacobsen syndrome (20). Previous work has demonstrated that ETS1 is present in two specific cell lineages during the formation of the mouse heart, namely the neural crest and the endocardium (28). These findings indicate that abnormalities in either one or both of these cell types may cause the phenotypic manifestations of this cardiologic condition. Indeed, it is relevant to note that mutations in ETS1 that result in loss of function during the early stages of heart development in Drosophila melanogaster lead to a reduction in pericardial cells and an increase in the number of cardiomyocytes (29). Moreover, this route targets the posterior part of the heart tube, which can be likened to the left cardiac ventricle in a heart of mammals. However, this selective effect on a posteriorly positioned subgroup of cardiomyocytes lacks a defined molecular foundation, at least sofar. Downregulation of ETS1 was carried out in the cardiac mesoderm in Xenopus. It resulted in a “ventricular phenotype” that closely resembled the morphological characteristics of the disease. Explicitly, there was an increase in the thickness of the ventricular wall, marked by an enlargement of the “compact myocardial zone” and a near-total absence of the “trabecular myocardial layer”. The observed phenomenon was linked to a depletion of cells localized at the endocardium (30). Conversely, rather than a thicker ventricular muscular layer (thick myocardium), the suppression of ETS1 in the cardiac mesoderm resulted in a very young, slim-walled and hypoplastic cardiac ventricle, along with a variation exhibiting a probably intermediate phenotype. In this last pattern, the ventricular muscular wall exhibited partial thinning and thickening. The status of the “growth-arrested heart” as a separate phenotypic or a prelude to the emergence of the “hypoplastic left heart-like” ventricular phenotype remains uncertain. Suppressed in the zone of multipotent stem cells located at the side of the neural tube and proximal to the epidermal layer following the neurulation (“neural crest”), a decrease in the concentrations of ETS1 resulted in abnormalities of the aorta and, simultaneously, of the outflow tract but had no impact on the formation of the ventricles. This data is consistent with the findings in individuals, which state that lesions can affect the aortic valve without affecting the development of the ventricles. Aggregately, these findings suggest the presence of one or more elements derived from the cardiac mesoderm that regulate the growth and differentiation of cells in the compact myocardial layer and trabecular myocardial layer of the ventricular wall of the heart. These factors may also be necessary for the development of the endocardium. Mutations in the NOTCH1 gene have also been documented to correlate with the syndrome (19). Similar to “loss-of-function” mutations of ETS1 gene, mutations in the NOTCH1 gene were described as an augmentation of cardiac myocytes in Drosophila melanogaster (fruit fly), accompanied by a total depletion of pericardium cells. The outcome is a very atypical “bifid heart” characterized by tightly clustered cell clusters and a reduced chamber capacity (31). Reports have also indicated a mutation in the NKX2-5 gene in connection with the condition (14). Deleting this gene conditionally in the mouse ventricle results in a cardiomyopathic phenotype characterized by increased expression of BMP10. Overexpressing BMP10, specifically in the ventricles of the mouse model, leads to an increase in ventricular trabeculations (“non-compaction”), resulting in a reduction in chamber capacity. However, this response is accompanied by a thin out compact layer (32). Thus, in this animal model, cavitary cardiac hypoplasia is caused by excessive growth of the trabecular layer rather than the compact layer without fibroelastosis. These findings have little similarity to the phenotype characterized by mitral stenosis observed in cases of HLHS. The upregulation of BMP10 in the left ventricle of mice, together with the findings after knocking down ETS1 in Xenopus, align with previous observations that elucidated the involvement of Hand2 in controlling the equilibrium between the compact element and the trabecular element of the rodent ventricular walls (30). The investigation demonstrated that the lack of Hand1 following the loss of Hand2 resulted in a reduction in left ventricular cardiac cavity, primarily attributed to the thickening of the mural components. Furthermore, the researchers detected aberrant expression of markers in the compact myocardium of the left ventricle but decreased markers in the trabecular layer (30).
Although there is considerable genetic evidence for HLHS, the exact genetic cause of these lesions is still not well known. HLHS remains genetically heterogeneous. There is evidence of incomplete penetrance for the C57Bl/6J-b2b635Clo/J (Ohia) mice. Two of the five homozygous mutations found in the Ohia line with two founder HLHS mutants are necessary for the disease: one is a missense mutation in Pcdha9, which encodes the cell-adhesion protein protocadherinA9, and the other is a splice-site mutation in Sap130, which encodes a Sin3A-associated protein in the histone deacetylase complex (33).
Anatomic pathology
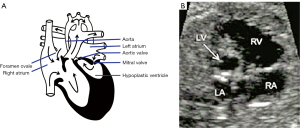
The analysis of the endocardium in HLHS remains critical in any gross and autopsy study (34). During fetal development, aortic stenosis is one of the first abnormalities to be detected in cases of HLHS (35). This implies that, in a certain group of patients with the syndrome, the presence of poor functioning of the aortic valve is a necessary condition for the formation of the aberrant left ventricle. In Figure 4, a dysfunctional left ventricle of the heart is depicted in the second trimester of gestation. This fetus exhibited the phenotypic characteristics of the disease during gestation (35). The precise processes responsible for the shift from the enlarged and dysfunctional left ventricle to the characteristic thick-walled ventricle are yet unknown. The key sonographic evidence comprises a diminutive, robust, hyperechoic left ventricle with limited contractile strength. A left ventricle that is either absent or small, together with an anterior mitral cardiac leaflet measuring 0.5 cm (5 mm) or less, is indicative of a hypoplastic ventricle of the left heart, an enlarged right ventricle with increased move of the tricuspid cardiac valve, lack of antegrade flow via the aortic valve, and poor movement of the aortic valve (36).
Additionally, there is a minor or not visible ascending aorta hypoplasia and minimal flow passing via it. An aortic root measuring 0.5 cm (5 mm) or less indicates the presence of atresia of the aortic valve, hypoplasia of the mitral valve with or without detectable flow, and impairment of mitral valve drive. Associated with the hypoplasia of the left heart, extracardiac abnormalities may manifest as 2-vessel cord, craniofacial (with or without central nervous system) anomalies, and gastrointestinal or gastrointestinal-genitourinary abnormalities.
It is logical to propose a first cessation in the development of the left cardiac ventricle compared to the right, subsequently accompanied by an increase in the thickness of the left ventricular wall of the heart. Relevantly, Snider et al. (37) investigated a possible direct involvement of the endocardium in the formation of the left ventricle. A genetic ablation of the endocardium was successfully performed during the development of the mouse heart. Diphtheria toxin was used to create timed and targeted death of the endocardial cells. These cells expressed an endocardial-specific CRE driver specifically designed for this purpose. By E11, the disturbance caused embryonic mortality by arresting the development of the left ventricle. In that setting, they display an outgrowth halt in the presence of a “thin-walled” and tiny cardiac ventricle on the left, these embryos resemble a subgroup of ETS1 cardiac mesodermal knockdown embryos reported above. Impairment of endocardium layer function resulting in an initial halt of development of the evolving left ventricle could be a crucial first stage in the development of the condition.
The observed outcome may be attributed to a fundamental genetic abnormality that results in the impairment of endocardial function. Furthermore, a deficiency in the formation of the valvar could result in endocardial damage and subsequent loss of function, as previously explained. Experimental investigations conducted on rats have offered more evidence connecting compromised hemodynamics and decreased ventricular function to abnormalities in the heart muscle. Thus, surgically produced aortic regurgitation caused enlargement of the left ventricle. It has been demonstrated to result in endocardial damage and endocardial fibroelastosis (38). Research on growing chicks has shown that ligating the left atrium can reduce flow across the mitral valve, leading to left ventricular hypoplasia (11). A recent study demonstrated that the reduced blood flow resulted in hypoxia (39).
Consequently, this led to the development of endocardial fibroelastosis, after which cardiomyocytic hyperplasia occurred. These findings align more closely with the observed characteristics in the clinical situation. Previous research on zebrafish has shown that reduced left ventricular activity during early development hinders the growth of both the atrioventricular and arterial valves (40). Both gross anatomy and microscopy are advantageous in illustrating the “slit-like” hypoplasia of the left ventricle, and step sections may be necessary in some instances.
A mounting body of evidence suggests that a fundamental abnormality in ventricular development causes the hypoplasia of the left ventricle. It has been suggested that, at least in certain circumstances, the chamber hypoplasia results from the cardiomyocytes’ hyperplasia. Within this context, HLHS might be considered a type of cardiomyopathy that occurs in newborns. Remarkably, the left ventricle cardiomyocytes of HLHS show an electrophysiological anomaly in their cardiomyocyte development, including short myofilament bundles, increased branching, and poorly defined Z bands. Alterations in the HLHS right ventricle were comparable, albeit less severe. Moreover, the mitochondria in the HLHS left ventricle were smaller, changed form, and had a low-density matrix with fewer cristae. Taken as a whole, these findings pointed to an issue with mitochondrial maturation. In this article, I further explore the involvement of the endocardium in the progression of ventricular hypoplasia, therefore offering a potential explanation for the morphological alterations observed in the disease as a result of compromised blood flow to the developing ventricle. These findings may support a new paradigm for the complicated genetics of this congenital heart defect. They may reveal that HLHS can originate genetically in a combinatorial approach (33).
Investigation of neural crest cells in HLHS
The parallel expression of ETS1 in both the neural crest and endocardium has suggested the potential involvement of either or both of these lineages in the development of the syndrome. Humans with HLHS are found to have a 117-fold increased incidence of Hirschsprung’s disease, an illness affecting the neural crest (41). This observation implies that the correlation above may be attributed to compromised functioning of the neural crest. Notably, studies with ETS1 deletion mice did not yield the expected characteristics of HLHS. Instead, the absence of ETS1, which has a particularly high penetrance, resulted in rodents (mice) with a right ventricle of the heart with two outlets. This data was contingent upon the genetic lineage.
Furthermore, it has been demonstrated that parentage of individuals with the syndrome experience a much higher single dilation of the ascending aorta, a quite well recognized result of “cardiac neural crest” proliferation (42). Consistent with this finding is the markedly higher occurrence of atypical aortic valves in immediate family members of individuals diagnosed with HLHS. The observations indicate that the poor function of neural crest cells may play a role in forming the aortic valve and arch, suggesting a possible contribution to the condition’s occurrence.
Coronary status and cardiomyocyte proliferation
There may be also some coronary arterial hypoplasia in HLHS (34). Patients diagnosed with the syndrome present with mitral stenosis often exhibit thickening of the left ventricular walls, but their left coronary artery system is usually somewhat small. This data stands in opposition to the enlarged and dilated left coronary artery order described in patients with hypertrophic cardiomyopathy. The latter observation is undoubtedly an adaptive reaction necessary to sustain a sufficient artery source to the significantly stiffened myocardium. The etiology of the “relative coronary artery hypoplasia” in the presence of mural thickened setting observed in HLHS is yet unclear.
Nevertheless, genetically modified animal models for genes linked to the condition exhibit a profusion of left ventricular mural cardiomyocytes. Under these circumstances in fetal development, the small left coronary artery system might result in a relative imbalance between hoard and necessity, guiding to impaired blood flow and reduced oxygen supply to the heart. A multitude of research has provided evidence that hypoxia serves as a trigger for cardiomyocytic hyperplasia (43). Therefore, the small left coronary artery system, which causes a discrepancy between the supply and demand in the growing ventricular walls, can worsen the cardiomyocytic hyperplasia phenomenon. Studies performed on Twin Reversal Arterial Perfusion sequence have been pillar to suggest hypoxia as a major culprit of congenital heart defects, at least in some dysmorphology constellations (44-46). Moreover, studies involving animal models in gastrointestinal research have also been pointing out to hypoxia as a major contributing factor in some neonatal disorders (27,47-53).
New mechanistic concepts in HLHS
A growing unifying theme is indicated by the data in the genetically modified animal models for ETS1, Hand1, as well as NKX2.5 and NOTCH1, four well studied genes known to be linked to the condition, as shown in humans. An imbalance in the normal growth of the compact layer and the trabecular layer of the ventricular walls of the heart causes hyperplasia of the cardiomyocytes in the early stages of cardiac development. Furthermore, the ETS1 and NOTCH1 mutations resulted in the depletion of a neighboring cell population, namely the pericardium-located cells in Drosophila melanogaster and the endocardium-located cells in Xenopus. This observation implies that these two genes have a role in maintaining equilibrium and determining the destinies of the two classes of cells. Prior investigations have shown that Etsrp/Etv2, a member of the ETS family, is necessary for maintaining equilibrium in determining the destinies of endocardial cells rather than cardiomyocytes. Depletion of Etsrp/Etv2 is associated with a reduction in endocardial cells and an increase in cardiac myocytes (54). Collectively, these scientific investigations indicate a causal relationship between the depletion of endocardial cells, resulting in diminished endocardial function, and the development of cardiomyocytic hyperplasia. This may be accomplished by directly influencing the destiny of the cellular populations or by disrupting the communication between the endocardial and myocardial cells. Alternatively, the link may indicate hemodynamic alterations resulting from a fundamental abnormality in the formation of either the aortic valve or mitral valve of the heart. Insufficient blood flow into the growing left ventricle due to a defect in the mitral valve may lead to damage and malfunction of the heart muscle, therefore supporting the flow theory in explaining the illness. An alternative hypothesis is that mutations in genes that impact the growth of cardiomyocytes may indicate a self-regulating mechanism of the cardiomyocytes. This would comprise downstream pathways specific to cardiomyocytes controlled by endocardial signals. This “two-hit” paradigm, which considers both valvar and ventricular development, would result in a self-perpetuating loop where valvar development hampers ventricular growth, and conversely, deficient ventricular development worsens already impaired valvar development. The ultimate objective might be identifying the phenotypic variation of the syndrome observed in mitral stenosis. Regardless of the scenario, a certain element or several components must exist that make the ventricle more likely to develop when there is endocardial dysfunction. Identifying the underlying reasons why, in certain instances, the development of the ventricles during fetal life can continue without any abnormalities despite the presence of aortic stenosis could offer a valuable understanding of how to prevent the syndrome itself. Although initially, the outcomes of therapeutic fetal balloon angioplasty were unsatisfactory (12), looking back, they are not unexpected and have the potential to provide valuable insights. These results are expected to align with the predictions from the animal models specified earlier. Therefore, future research should investigate the biocellular makeup of the ventricular walls not only at light microscopy, but also at electron microscopy level in specimens exhibiting the clinical characteristics of the disease. It is necessary to determine the frequency at which the thickening of the walls is caused by cardiomyocytic hyperplasia rather than an increase in the proliferation of other cell types. Once it is established that hyperplasia is present, it is necessary to determine if there is a disturbance in the equilibrium between the growth of the compact and trabecular zones. An unresolved issue of significance is how a dilated, slim-walled, and dysfunctional left ventricle of the heart, which is observed during early fetal development, progresses into the characteristic stiffened left ventricle observed in the variation of the condition with stenosis of the cardiac mitral valve. Another significant matter is the correlation between the valves and the left ventricle formation. The etiology of the observed diversity in human phenotypes remains still unidentified. A full understanding of the molecular connections between poor valvar and ventricular development necessitates more study in this crucial field. Finally, it will be crucial to determine how the controlling genetic nets impact individuals with the disease. Moreover, in our opinion, researchers should target the functions of the “cardiac neural crest” and the “coronary artery system”, which are probably two not adequately studied in depth settings. These topics are worth investigating. Complementing these investigations will be necessary to elucidate the well-established intricate genetics. There is knowledge of a causal genetic mutation in only few cases.
One may argue that the study by Liu et al. (33) has provided further insight into this possible correlation. Nevertheless, as observed in humans, none of the mice mutants documented by these authors exhibited the properly classic phenotypic characteristics of HLHS. Despite the presence of aortic atresia and a hypertrophied left ventricle, the hearts did not display the characteristics of HLHS. This is because none of the models demonstrated the “integrity” of the ventricular septum when the atrio-ventricular (cardio-cardial) and ventriculo-arterial (cardio-vasal) connections were concordant. On the other side, we believe that the first animal models of HLHS were recovered with the help of mouse forward genetics. Despite HLHS mutant mice are not perfect, the authors confirmed that HLHS is caused by mutations in Sap130 and Pcdha9. A two-locus hypothesis that had been suggested for HLHS is compatible with this digenic etiology. Animal studies showed that aortic valve abnormalities are caused by mutations in protocadherin, whereas left ventricular hypoplasia is caused by Sap130, which disrupts cardiomyocyte proliferation and differentiation.
The examination of the study by Liu et al. (33) reveals that the hypoplastic aorta originates from the upper chamber of the right ventricle of the heart. The left ventricle seems to not exhibit any of the expected fibroelastotic alterations characterized in the human variant phenotype characterized by mitral valve stenosis and aortic valve atresia (33). Presently, there are no other mouse models available that accurately reproduce better the “classical” forms of HLHS seen in affected human individuals. The reason for this is that the evidence obtained from the analysis of human variations and the results from fetal ultrasonography (cardiac echography) already mentioned may give credence to the conclusion that the condition is an entity acquired during fetal development. More precisely, the alterations in the structure of the hearts affected by mitral stenosis occur when the aortic root becomes attached to the evolving left ventricle and the specific embryonic interventricular pathway is closed. While it is theoretically feasible to track these changes in a mouse model, it is crucial to demonstrate that these changes specifically occurred after the definitive closure of the interventricular communication at embryonic level, despite disputes and controversies (33,55,56). I acknowledge the substantial verification readily available to substantiate that the condition has a hereditary element. It would be premature to assume that investigations like those documented by Liu et al. (33) have definitively shown the connection, but they are and continue to remain highly promising (2). Therefore, the ongoing endeavors of the Pediatric Cardiac Genomics Consortium (20) have revealed a limited number of probable genes responsible for the syndrome. Subsequent investigations should ascertain whether the remaining instances, now classified as idiopathic, are caused by disruptions in other genes within the established pathways. Should this not be the situation, they will undoubtedly result in the discovery of new routes and, therefore, establish the foundation for fresh fields of study. The “Todd and Karen Wanek Family Program” for HLHS is now undertaking further initiatives that have the potential to develop novel therapeutic strategies for the syndrome (57,58). Thus, there is still much to be understood regarding the development of HLHS.
The published research data discussed in this review demonstrates the intricate and multifaceted causes, such as genetic, epigenetic, and environmental factors, that probably need to coincide to produce the characteristics indicative of HLHS observed in clinical settings. Furthermore, they highlight the obstacles in creating animal models that precisely simulate the disease. While the details of most cases remain unclear, there is reason to be optimistic about gaining a deeper knowledge of the molecular mechanisms behind this catastrophic illness.
Clinical status and therapeutical approaches
Untreated, the illness is irreversibly lethal, often occurring during the first weeks after birth. The surgical treatment is of a palliative nature, involving three surgical operations during the initial years of life. The initial operation, “Norwood surgery”, is conducted during the newborn period. This is then followed by creating a “Glenn anastomosis” between the age of 4 and 6 months. The conversion to the “Fontan circulation” is often accomplished by the age of 3 years. These procedures aim to restore the heart to operate as a functional two-chambered organ, where the morphologically exhibiting right ventricle functions as the systemic cardiac ventricle. Only a minority of newborns may undergo heart transplantation as a viable treatment option. The restricted availability of donor hearts makes this treatment impractical for the majority of newborns with abnormalities. Collectively, the condition is the primary factor contributing to illness and death in newborns with congenital heart disease. The long-term survival outlook is uncertain. This is because, in time, the systemic right ventricle may experience failure, resulting in early death if no further treatments are provided. Thus, heart transplantation may ultimately become the sole feasible alternative for ensuring long-term survival. Nevertheless, the criteria for determining the appropriate time to list individuals for cardiac transplantation are still debatable and frequently lack clarity. Indeed, it is not unusual for people experiencing right heart failure to succumb before getting a donor’s heart. Therefore, the syndrome and the accompanying treatment tremendously hardship families. It might lead to substantial work time loss for the parents and exceptional psychological stressing factors. Considering all aspects, the syndrome is the most costly congenital cardiac condition, with an estimated yearly direct payment of about $1 billion in the United States of America (USA) for this ailment (59). There is no doubt that left untreated, HLHS is a universally lethal variant, accounting for 22% of fatalities caused by congenital heart disease during the first year of life. The prognosis for the HLHS is unfavorable, with a survival rate of 40–55% following prenatal diagnosis (60,61). The use of prenatal sonological diagnosis has demonstrated a superior outcome in comparison to neonates who are not diagnosed before delivered (62). Ultrasonography during the second trimester shows a tiny, dysfunctional left cardiac ventricle as well as significant aortic stenosis with or without severe coarctation of the aortic vessel. Images captured in real-time will probably clearly show the blocked flow and impaired ability of the left ventricular wall of the heart to contract (62). These fetuses, when monitored throughout gestation, may exhibit elevated heart wall echogenicity indicating endocardial fibroelastosis, and the left ventricle of the heart reduces in size compared to the typically developing right ventricle of the heart (63).
Dilation of the aortic valve during pregnancy has been suggested as a treatment for HLHS caused by severe aortic stenosis and intrauterine cardiac surgery may be considered a future procedure plan. In fact, this procedure should be offered and allows parents to elect intrauterine procedures if they choose to proceed with the pregnancy. The study conducted by Stoll et al. shown that the sensitivity of sonographic identification for isolated HLHS was 61.9% (64). Generally, the cardiac abnormalities that impact the dimensions of the ventricles have the highest rate of discovery. Different investigations have reported sensitivities of 36.6% and 37% for prenatal sonographic diagnosis, and this data may be considering alarming, particularly if an interruption of pregnancy is suggested (64).
Indeed, HLHS often manifests within the first week of life. It is characterized by indications of reduced systemic blood flow caused by the narrowing of the ductus arteriosus (Botalli), which results in a decrease in the resistance found in the pulmonary vessels. These newborns endure their abnormality for a few days until the ductus arteriosus may be fully dilated. The ductus constricts when the arterial pressure drops, leading to severe metabolic acidemia. Left untreated, nearly all of the afflicted newborns succumb within 6 weeks. Only the determination of the karyotype and the search for related abnormalities are necessary active obstetrical interventions during pregnancy. Adequate prenatal diagnosis is crucial for providing pregnancy counseling and preparing for delivery, given the seriousness of this disorder and the need for skilled surgical intervention. Prenatal diagnosis facilitates the prevention of “ductal shock” by preventing the closure of the ductus arteriosus after birth, sometimes achieved by implementing the administration of prostaglandin E14. Proposed palliative surgical interventions such as the modified “Norwood surgery”, bidirectional cavo-pulmonary shunt, modified “Fontan technique”, aortic valvuloplasty, and cardiac transplantation have shown to enhance the continued existence of these youngsters. The initial therapeutic strategy involves graded surgical palliation, which, if proved ineffective, may eventually necessitate heart transplantation.
In summary, patients with HLHS have primarily been managed with the phased surgical method, which culminates in the Fontan circulation, since its introduction over 40 years ago (65). Protecting the pulmonary vascular bed from pulmonary hypertension and restoring reliable systemic and pulmonary blood flow are the goals of the first stage of the Norwood protocol. A Damus-Kaye-Stansel (DKS) connection is used to repair the neo-aorta during the first week of life. This is achieved by splitting the main pulmonary artery and anastomosing it to the ascending aorta. A right ventricle-to-pulmonary artery conduit or a modified Blalock-Taussig shunt supplies blood to the lungs. Additionally, an atrial septectomy is done to make sure the right ventricle may fill up without any restrictions. The right ventricle is then attached to the neo-aorta. Patients at extremely high risk, such as those with major extracardiac malformations, low birth weights, premature births, or who are not yet ready for the Norwood Stage 1 treatment, may benefit from a hybrid approach. This method integrates off-pump surgery with interventional cardiac catheterization. It entails atrial septectomy or percutaneous balloon atrial septostomy, banding of the pulmonary arteries on both sides, and stenting of the ductus arteriosus. The two methods differ mostly in the perfusion of the coronary and cerebral circulations. The second type uses a stented ductus arteriosus to supply blood retrogradely to the coronaries and brain. Thus, it is crucial to closely monitor the patient because stenosis at the point of the ductus arteriosus connecting to the native aorta can cause retrograde aortic arch obstruction. The second stage of the Norwood technique is a bidirectional Glenn, which is typically done between 4 and 6 months of age. This operation lessens the volume stress on the systemic right ventricle and improves the reliability of pulmonary blood flow by anastomosing the superior vena cava to the pulmonary artery. Finally, in Stage 3 of the Norwood protocol, the Fontan procedure is performed to entirely isolate the pulmonary circulation from the systemic circulation. This is achieved by directly connecting the inferior vena cava to the pulmonary artery, either by an intracardiac or extracardiac conduit. The typical age for the procedure is 2 or 3 years old.
Discussion
HLHS is a form of univentricular heart characterized by a small left ventricle and a functional right ventricle (65,66). The main pulmonary artery arises from the right ventricle and directs blood to the pulmonary vasculature. The primary pulmonary artery facilitates systemic blood flow via the intermediary ductus arteriosus. During the prenatal stage, elevated pulmonary vascular resistance ensures that cardiac output effectively supplies circulation to the brain, descending aorta, and coronary arteries via the ductus arteriosus. Following birth, a decrease in pulmonary vascular resistance results in cardiac output being mostly allocated to the pulmonary vasculature, leading to reduced systemic circulation via the ductus arteriosus and a small left ventricle. It may result in hypoxia and metabolic acidosis, contingent upon the severity of compromised systemic blood circulation. Conversely, increased pulmonary blood flow elevates the risk of pulmonary edema (66-69). The postnatal existence of an interatrial defect (patent foramen ovale or atrial septal defect) is crucial for systemic blood oxygenation and left atrial decompression by facilitating the flow of pulmonary venous blood from the left atrium to the right heart chambers. This setting may be complicated by thrombotic and infectious events and needs to be closely monitored (66,67,70).
The inefficiency of the left ventricle places the right ventricle at danger of dysfunction as a systemic ventricle. The inferior texture of right ventricular myocardium, the challenge posed by the robust systemic circulation, and the lack of diastolic suction function of the left ventricle are contributing factors to right ventricular failure. Moreover, this univentricular right ventricle is prone to hypertrophy, leading to subsequent myocardial fibrosis and dysfunction. In pressure overload conditions, the right ventricle exhibits a diminished ability for angiogenesis compared to the left ventricle, attributable potentially to variations in gene expression. This results in diminished myocardial perfusion and exacerbation of right ventricular dysfunction. The pathological burden in the right ventricular myocardium may impair right ventricular contractility. Fibrosis, apoptosis, reduced antioxidant response, and reliance on glycolytic metabolism rather than more efficient oxidative mitochondrial metabolism are further factors associated with right ventricular failure. Stem-cell therapy has focused on the treatment of right ventricular failure in HLHS (67-69,71).
HLHS remains a very complex genetic disease. Recognizing the associated gene defects in HLHS is a prophecy for future therapeutic strategies. Whole genome sequencing is the regular method of gene defect identification. Few genes, including NOTCH1, NKX2-5, ETS1, MYH6, lipoprotein receptor-related protein 2 (LRP2), CELSR1, MYO18B, PTPRB, and KMT2D/KDM6A (in Kabuki syndrome), have been recognized in HLHS, although they have also been identified in other congenital heart diseases (72,73). Because of the profound genetic heterogeneity of HLHS, its genetic evaluation is more accessible through clinical studies based on the familial study design. Further, the filtering process is required to find related alleles of HLHS because an individual genome contains millions of rare variants. Defective mitochondrial metabolic pathways (carnitine palmitoyl-transferase, tricarboxylic acid cycle, and Ca2+ signaling pathways) are the potential causes of heart failure in single ventricular anomaly (72,74-79). Decreased myocardial cell survival and contractility correlate with altered mitochondrial respiration and oxidation and heterogeneity in the cellular phenotype could be responsible for the wide clinical spectrum of the disease in HLHS patients (72,75,80). Despite the normal size of the right ventricle in HLHS patients, it has mitochondrial defects similar to the left ventricle but of lower severity (77,80-83). This may be an essential factor for the right ventricular failure after palliative surgery. These findings may be critical to experiment mitochondrial-targeted therapies for the improvement of the myocardial cell dysfunction.
Key findings
The studies analyzed indicated that HLHS is one of the most complex congenital heart defects, characterized by a small or hypoplastic left ventricle, small or hypoplastic left-sided heart structures, and a dominant right ventricle. The Fontan circulation and the phased surgical technique that it entails have been the cornerstones of HLHS patient care since its debut some 40 years ago. Although there is considerable genetic evidence for HLHS, the exact genetic cause of these lesions is still not well known. HLHS remains genetically heterogeneous. There is evidence of incomplete penetrance for the C57Bl/6J-b2b635Clo/J (Ohia) mice. Two of the five homozygous mutations found in the Ohia line with two founder HLHS mutants are necessary for the disease: one is a missense mutation in Pcdha9, which encodes the cell-adhesion protein protocadherinA9, and the other is a splice-site mutation in Sap130, which encodes a Sin3A-associated protein in the histone deacetylase complex.
The mechanisms behind the syndrome are a subject of contention, primarily attributed to the notable phenotypic diversity of the disorder. Commonly, it is believed that the reduced growth of the left ventricle is caused by reduced blood flow during a crucial phase of the early ventricular development of the heart. Progress in research has been impeded by the absence of genetically modified animal models that accurately replicate human disease, although the Ohia mice seem to currently be the best animal model.
The literature pointed to an issue with mitochondrial maturation in the setting of HLHS. In this article, we further explore the involvement of the endocardium in the progression of ventricular hypoplasia, therefore offering a potential explanation for the morphological alterations observed in the disease as a result of compromised blood flow to the developing ventricle. These findings may support a new paradigm for the complicated genetics of this congenital heart defect. HLHS can originate genetically in a combinatorial approach.
Strengths and limitations
This paper is probably one of the most comprehensive literature reviews on HLHS, including a wide range of studies from different clinical and research groups, providing an extensive overview of the role of genetics in HLHS. This breadth of knowledge allows for a more complete understanding of how genetic factors influence with epigenetic factors the development of this syndrome. Additionally, this scoping review identified gaps and controversies in the existing literature, such us the evidence of incomplete penetrance for the C57Bl/6J-b2b635Clo/J (Ohia) mice. Two of the five homozygous mutations found in the Ohia line with two founder HLHS mutants are necessary for the disease: one is a missense mutation in Pcdha9, which encodes the cell-adhesion protein protocadherinA9, and the other is a splice-site mutation in Sap130, which encodes a Sin3A-associated protein in the histone deacetylase complex. The mechanisms behind the syndrome are a subject of debate in the scientific community, primarily attributed to the notable phenotypic diversity of the disorder. These insights associated with the mitochondrial studies, which our research group are targeting, are valuable for guiding future research and interventions, helping to direct resources and efforts towards areas that could have the potential to grow in the future.
The limitations of the study are intrinsically associated with the scoping review. This study did not assess the quality of included studies other than an ad hoc methodology. Although gray literature was consulted, the major focus on published literature might have missed relevant unpublished or non-peer-reviewed studies, limiting the scope of findings. Although HLHS may affect all ethnics and groups, and despite the diversity of studies included, certain demographic groups may still be underrepresented. Epidemiological studies in the future should be able to fill this gap.
Comparison with similar research
The findings of this scoping review align with the most updated research on HLHS with the difference that this study implemented a discourse on the contentious Ohia rodents and their significance for the genetics factors of HLHS. Moreover, this study collected evidence on several areas of HLHS suggesting that this scoping review is probably one of the most thorough reviews of the literature. Finally, the identification of mitochondrial abnormalities in HLHS has raised low interest so far, but we hope that the current data may stimulate further research and international collaboration toward this direction.
Explanations of findings
The findings of this scoping study rely on the solidity of the database search and the screening of the gray literature. However, the knowledge of this complex and complicate congenital heart defect is limited, because of the genetic heterogeneity and the lack of probably solid experimental animals.
Implications and actions needed
The studies and the findings raise the question of further research focusing on the genetic and epigenetic factors. It is necessary to polarize the interest of charities and research grant agencies toward the HLHS and the modality to act early with both “intrauterine” and/or “ex utero” surgery. While there is no standard “intrauterine” surgery to cure HLHS, fetal interventions like fetal aortic valvuloplasty are sometimes performed to improve blood flow and potentially prevent or delay the need for complex postnatal surgeries. Fetal aortic valvuloplasty involves inserting a catheter into the baby’s heart through the mother’s abdomen to dilate the aortic valve using a balloon. Other potential intrauterine interventions may include creating a functional ventricular septal defect to improve blood flow and left ventricle development. The benefits of fetal intervention include an improved blood flow and some potential for biventricular circulation. Although fetal interventions are not a cure for HLHS, they can improve outcomes and reduce the need for complex postnatal surgeries. In this sense, the role of a pediatrician and/or a pediatric pathologist is crucial in supporting the diffusion of surgical centers with experience in fetal cardiac surgery. The implementation of robotic-assisted surgery for several areas may gain support by healthcare providers, particularly if artificial intelligence and machine learning protocols are coupled.
Conclusions
HLHS, a complicated collection of cardiac abnormalities, can be identified during prenatal assessment using ultrasonography. Intrauterine cardiac intervention has been established in some centers. Nevertheless, the search for a major culprit, either genetic or environmental-related, is far from the end. There are a few genes that have been associated with this cardiologic condition, but chronic hypoxia has been suggested as a contributing factor for some data that have been collected over the years using some animal models. There are individuals with the syndrome displaying a phenomenon known as “non-compaction” of the right ventricle, similar to the left-ventricle non-compaction (84). Morphologic studies targeting the trabeculation of the ventricles may also be useful to identify patterns for further molecular genomic studies. My research group and I hope that transcriptomics and subsequent single cell data analysis may be useful for the identification of a factor, which may be able to be corrected early during gestation.
Acknowledgments
The author would like to recognize first the parents who suffered from the fetus or child affected with hypoplastic left heart syndrome, and second all physicians, nurses, and other allied healthcare workers who are responsible for the care of parents affected with congenital heart syndrome.
Footnote
Reporting Checklist: The author has completed the PRISMA-ScR reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-24-367/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-24-367/prf
Funding: The research has been funded by
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-24-367/coif). C.M.S. serves as an unpaid editorial board member of Translational Pediatrics from March 2024 to February 2026. C.M.S. declares that he receives royalties from Springer Publisher and other publishers. All royalties go to pediatric charities. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gehrmann J, Krasemann T, Kehl HG, et al. Hypoplastic left-heart syndrome: the first description of the pathophysiology in 1851; translation of a publication by Dr. Bardeleben from Giessen, Germany. Chest 2001;120:1368-71. [Crossref] [PubMed]
- Grossfeld P, Nie S, Lin L, et al. Hypoplastic Left Heart Syndrome: A New Paradigm for an Old Disease? J Cardiovasc Dev Dis 2019;6:10. [Crossref] [PubMed]
- Lev M. Pathologic anatomy and interrelationship of hypoplasia of the aortic tract complexes. Lab Invest 1952;1:61-70. [PubMed]
- Noonan JA, Nadas AS. The hypoplastic left heart syndrome; an analysis of 101 cases. Pediatr Clin North Am 1958;5:1029-56. [Crossref] [PubMed]
- Haas NA, Zelle M, Rosendahl W, et al. Hypoplastic left heart in the 6500-year-old Detmold Child. Lancet 2015;385:2432. [Crossref] [PubMed]
- Joanna Briggs Institute Reviewers’ Manual 2015. JBI; 2015:24 p.
- Haring MPD, Cuperus FJC, Duiker EW, et al. Scoping review of clinical practice guidelines on the management of benign liver tumours. BMJ Open Gastroenterol 2021;8:e000592. [Crossref] [PubMed]
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018;169:467-73. [Crossref] [PubMed]
- Crucean A, Alqahtani A, Barron DJ, et al. Re-evaluation of hypoplastic left heart syndrome from a developmental and morphological perspective. Orphanet J Rare Dis 2017;12:138. [Crossref] [PubMed]
- Tchervenkov CI, Jacobs JP, Weinberg PM, et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol Young 2006;16:339-68. [Crossref] [PubMed]
- deAlmeida A, McQuinn T, Sedmera D. Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ Res 2007;100:1363-70. [Crossref] [PubMed]
- Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation 2004;110:2125-31. [Crossref] [PubMed]
- Slough J, Cooney L, Brueckner M. Monocilia in the embryonic mouse heart suggest a direct role for cilia in cardiac morphogenesis. Dev Dyn 2008;237:2304-14. [Crossref] [PubMed]
- Elliott DA, Kirk EP, Yeoh T, et al. Cardiac homeobox gene NKX2-5 mutations and congenital heart disease: associations with atrial septal defect and hypoplastic left heart syndrome. J Am Coll Cardiol 2003;41:2072-6. [Crossref] [PubMed]
- Xu JH, Gu JY, Guo YH, et al. Prevalence and Spectrum of NKX2-5 Mutations Associated With Sporadic Adult-Onset Dilated Cardiomyopathy. Int Heart J 2017;58:521-9. [Crossref] [PubMed]
- Gardner MM, Cohen MS. Clinical findings in right ventricular noncompaction in hypoplastic left heart syndrome. Congenit Heart Dis 2017;12:783-6. [Crossref] [PubMed]
- Bohlmeyer TJ, Helmke S, Ge S, et al. Hypoplastic left heart syndrome myocytes are differentiated but possess a unique phenotype. Cardiovasc Pathol 2003;12:23-31. [Crossref] [PubMed]
- Gaber N, Gagliardi M, Patel P, et al. Fetal reprogramming and senescence in hypoplastic left heart syndrome and in human pluripotent stem cells during cardiac differentiation. Am J Pathol 2013;183:720-34. [Crossref] [PubMed]
- Garg V, Muth AN, Ransom JF, et al. Mutations in NOTCH1 cause aortic valve disease. Nature 2005;437:270-4. [Crossref] [PubMed]
- Glessner JT, Bick AG, Ito K, et al. Increased frequency of de novo copy number variants in congenital heart disease by integrative analysis of single nucleotide polymorphism array and exome sequence data. Circ Res 2014;115:884-96. [Crossref] [PubMed]
- Grossfeld P. ETS1 and HLHS: Implications for the Role of the Endocardium. J Cardiovasc Dev Dis 2022;9:219. [Crossref] [PubMed]
- Srivastava D, Gottlieb PD, Olson EN. Molecular mechanisms of ventricular hypoplasia. Cold Spring Harb Symp Quant Biol 2002;67:121-5. [Crossref] [PubMed]
- Homsy J, Zaidi S, Shen Y, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 2015;350:1262-6. [Crossref] [PubMed]
- Sifrim A, Hitz MP, Wilsdon A, et al. Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing. Nat Genet 2016;48:1060-5. [Crossref] [PubMed]
- Ferencz C, Boughman JA, Neill CA, et al. Congenital cardiovascular malformations: questions on inheritance. Baltimore-Washington Infant Study Group. J Am Coll Cardiol 1989;14:756-63. [Crossref] [PubMed]
- Schulkey CE, Regmi SD, Magnan RA, et al. The maternal-age-associated risk of congenital heart disease is modifiable. Nature 2015;520:230-3. [Crossref] [PubMed]
- Kumar P, Morton JS, Shah A, et al. Intrauterine exposure to chronic hypoxia in the rat leads to progressive diastolic function and increased aortic stiffness from early postnatal developmental stages. Physiol Rep 2020;8:e14327. [Crossref] [PubMed]
- Ye M, Coldren C, Liang X, et al. Deletion of ETS-1, a gene in the Jacobsen syndrome critical region, causes ventricular septal defects and abnormal ventricular morphology in mice. Hum Mol Genet 2010;19:648-56. [Crossref] [PubMed]
- Alvarez AD, Shi W, Wilson BA, et al. pannier and pointedP2 act sequentially to regulate Drosophila heart development. Development 2003;130:3015-26. [Crossref] [PubMed]
- Nie S, Bronner ME. Dual developmental role of transcriptional regulator Ets1 in Xenopus cardiac neural crest vs. heart mesoderm. Cardiovasc Res 2015;106:67-75. [Crossref] [PubMed]
- Hartenstein AY, Rugendorff A, Tepass U, et al. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development 1992;116:1203-20. [Crossref] [PubMed]
- Pashmforoush M, Lu JT, Chen H, et al. Nkx2-5 pathways and congenital heart disease; loss of ventricular myocyte lineage specification leads to progressive cardiomyopathy and complete heart block. Cell 2004;117:373-86. [Crossref] [PubMed]
- Liu X, Yagi H, Saeed S, et al. The complex genetics of hypoplastic left heart syndrome. Nat Genet 2017;49:1152-9. [Crossref] [PubMed]
- Sergi CM. Pathology of Childhood and Adolescence: An Illustrated Guide. Springer Berlin, Heidelberg; 2020.
- Feinstein JA, Benson DW, Dubin AM, et al. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol 2012;59:S1-42. [Crossref] [PubMed]
- Strzelecka I, Biedrzycka M, Karuga FF, et al. Seasonality of Hypoplastic Left Heart Syndrome and Single Ventricle Heart in Poland in the Context of Air Pollution. J Clin Med 2021;10:3207. [Crossref] [PubMed]
- Snider P, Simmons O, Wang J, et al. Ectopic Noggin in a Population of Nfatc1 Lineage Endocardial Progenitors Induces Embryonic Lethality. J Cardiovasc Dev Dis 2014;1:214-36. [Crossref] [PubMed]
- Shimada S, Robles C, Illigens BM, et al. Distention of the Immature Left Ventricle Triggers Development of Endocardial Fibroelastosis: An Animal Model of Endocardial Fibroelastosis Introducing Morphopathological Features of Evolving Fetal Hypoplastic Left Heart Syndrome. Biomed Res Int 2015;2015:462469. [Crossref] [PubMed]
- Pesevski Z, Kvasilova A, Stopkova T, et al. Endocardial Fibroelastosis is Secondary to Hemodynamic Alterations in the Chick Embryonic Model of Hypoplastic Left Heart Syndrome. Dev Dyn 2018;247:509-20. [Crossref] [PubMed]
- Goddard LM, Duchemin AL, Ramalingan H, et al. Hemodynamic Forces Sculpt Developing Heart Valves through a KLF2-WNT9B Paracrine Signaling Axis. Dev Cell 2017;43:274-289.e5. [Crossref] [PubMed]
- Ahola JA, Koivusalo A, Sairanen H, et al. Increased incidence of Hirschsprung's disease in patients with hypoplastic left heart syndrome--a common neural crest-derived etiology? J Pediatr Surg 2009;44:1396-400. [Crossref] [PubMed]
- Kelle AM, Qureshi MY, Olson TM, et al. Familial Incidence of Cardiovascular Malformations in Hypoplastic Left Heart Syndrome. Am J Cardiol 2015;116:1762-6. [Crossref] [PubMed]
- Nakada Y, Canseco DC, Thet S, et al. Hypoxia induces heart regeneration in adult mice. Nature 2017;541:222-7. [Crossref] [PubMed]
- Sergi C, Hentze S, Sohn C, et al. Telencephalosynapsis (synencephaly) and rhombencephalosynapsis with posterior fossa ventriculocele ('Dandy-Walker cyst'): an unusual aberrant syngenetic complex. Brain Dev 1997;19:426-32. [Crossref] [PubMed]
- Sergi C, Schmitt HP. Central nervous system in twin reversed arterial perfusion sequence with special reference to examination of the brain in acardius anceps. Teratology 2000;61:284-90. [Crossref] [PubMed]
- Sergi C, Schmitt HP. The vesicular forebrain (pseudo-aprosencephaly): a missing link in the teratogenetic spectrum of the defective brain anlage and its discrimination from aprosencephaly. Acta Neuropathol 2000;99:277-84. [Crossref] [PubMed]
- Cheung DC, Gill RS, Liu JQ, et al. Vasopressin improves systemic hemodynamics without compromising mesenteric perfusion in the resuscitation of asphyxiated newborn piglets: a dose-response study. Intensive Care Med 2012;38:491-8. [Crossref] [PubMed]
- Gill RS, Lee TF, Sergi C, et al. Early versus delayed cyclosporine treatment in cardiac recovery and intestinal injury during resuscitation of asphyxiated newborn piglets. Intensive Care Med 2012;38:1215-23. [Crossref] [PubMed]
- Hargitai B, Szabó V, Cziniel M, et al. Human brain of preterm infants after hypoxic-ischaemic injuries: no evidence of a substantial role for apoptosis by using a fine-tuned ultrasound-guided neuropathological analysis. Brain Dev 2004;26:30-6. [Crossref] [PubMed]
- Mojiri A, Alavi P, Lorenzana Carrillo MA, et al. Endothelial cells of different organs exhibit heterogeneity in von Willebrand factor expression in response to hypoxia. Atherosclerosis 2019;282:1-10. [Crossref] [PubMed]
- Pelletier JS, LaBossiere J, Dicken B, et al. Low-dose vasopressin improves cardiac function in newborn piglets with acute hypoxia-reoxygenation. Shock 2013;40:320-6. [Crossref] [PubMed]
- Sergi C. EPAS 1, congenital heart disease, and high altitude: disclosures by genetics, bioinformatics, and experimental embryology. Biosci Rep 2019;39:BSR20182197. [Crossref] [PubMed]
- Sergi C, Serpi M, Müller-Navia J, et al. CATCH 22 syndrome: report of 7 infants with follow-up data and review of the recent advancements in the genetic knowledge of the locus 22q11. Pathologica 1999;91:166-72. [PubMed]
- Palencia-Desai S, Kohli V, Kang J, et al. Vascular endothelial and endocardial progenitors differentiate as cardiomyocytes in the absence of Etsrp/Etv2 function. Development 2011;138:4721-32. [Crossref] [PubMed]
- Lo CW, Liu X, Gabriel GC, et al. Reply to 'Double-outlet right ventricle is not hypoplastic left heart syndrome'. Nat Genet 2019;51:198-9. [Crossref] [PubMed]
- Chaudhry B, Henderson D, Anderson R. Double-outlet right ventricle is not hypoplastic left heart syndrome. Nat Genet 2019;51:198. [Crossref] [PubMed]
- Hrstka SC, Li X, Nelson TJ, et al. NOTCH1-Dependent Nitric Oxide Signaling Deficiency in Hypoplastic Left Heart Syndrome Revealed Through Patient-Specific Phenotypes Detected in Bioengineered Cardiogenesis. Stem Cells 2017;35:1106-19. [Crossref] [PubMed]
- Burkhart HM, Qureshi MY, Peral SC, et al. Regenerative therapy for hypoplastic left heart syndrome: first report of intraoperative intramyocardial injection of autologous umbilical-cord blood-derived cells. J Thorac Cardiovasc Surg 2015;149:e35-7. [Crossref] [PubMed]
- Urencio M, Greenleaf C, Salazar JD, et al. Resource and cost considerations in treating hypoplastic left heart syndrome. Pediatric Health Med Ther 2016;7:149-53. [Crossref] [PubMed]
- Allan LD, Apfel HD, Printz BF. Outcome after prenatal diagnosis of the hypoplastic left heart syndrome. Heart 1998;79:371-3. [Crossref] [PubMed]
- Brackley KJ, Kilby MD, Wright JG, et al. Outcome after prenatal diagnosis of hypoplastic left-heart syndrome: a case series. Lancet 2000;356:1143-7. [Crossref] [PubMed]
- Allen RH, Benson CB, Haug LW. Pregnancy outcome of fetuses with a diagnosis of hypoplastic left ventricle on prenatal sonography. J Ultrasound Med 2005;24:1199-203. [Crossref] [PubMed]
- Hornberger LK, Need L, Benacerraf BR. Development of significant left and right ventricular hypoplasia in the second and third trimester fetus. J Ultrasound Med 1996;15:655-9. [Crossref] [PubMed]
- Stoll C, Dott B, Alembik Y, et al. Evaluation and evolution during time of prenatal diagnosis of congenital heart diseases by routine fetal ultrasonographic examination. Ann Genet 2002;45:21-7. [Crossref] [PubMed]
- Kačar P, Tamborrino PP, Iannaccone G, et al. Hypoplastic left heart syndrome (HLHS) becomes of age: Assessing the young adult with HLHS including the neoaorta/aortic arch. Int J Cardiol Congenit Heart Dis 2025;19:100555. [Crossref] [PubMed]
- Iskander C, Nwankwo U, Kumanan KK, et al. Comparison of Morbidity and Mortality Outcomes between Hybrid Palliation and Norwood Palliation Procedures for Hypoplastic Left Heart Syndrome: Meta-Analysis and Systematic Review. J Clin Med 2024;13:4244. [Crossref] [PubMed]
- Kreuzer M, Sames-Dolzer E, Klapper M, et al. The anatomic repair of recurrent aortic arch obstruction in children and adolescents. JTCVS Open 2024;19:215-22. [Crossref] [PubMed]
- Zielonka B, Bucholz EM, Lu M, et al. Childhood Opportunity and Acute Interstage Outcomes: A National Pediatric Cardiology Quality Improvement Collaborative Analysis. Circulation 2024;150:190-202. [Crossref] [PubMed]
- Albrahimi E, Korun O. Contemporary management of borderline left ventricle. Eur J Cardiothorac Surg 2024;66:ezae247. [Crossref] [PubMed]
- Mini N, Zartner PA, Schneider MBE. Transcatheter dilation and stenting of the modified blalock-taussig shunt in cyanotic heart diseases: points to consider. A single-center experience. Front Cardiovasc Med 2024;11:1445987. [Crossref] [PubMed]
- Choubey U, Srinivas V, Trivedi YV, et al. Regenerating the ailing heart: Stem cell therapies for hypoplastic left heart syndrome. Ann Pediatr Cardiol 2024;17:124-31. [Crossref] [PubMed]
- Yagi H, Xu X, Gabriel GC, et al. Molecular Pathways and Animal Models of Hypoplastic Left Heart Syndrome. Adv Exp Med Biol 2024;1441:947-61. [Crossref] [PubMed]
- Pfitzer C, Schmitt KRL, Benson WD. Human Genetics of Hypoplastic Left Heart Syndrome. Adv Exp Med Biol 2024;1441:937-45. [Crossref] [PubMed]
- Blue EE, White JJ, Dush MK, et al. Rare variants in CAPN2 increase risk for isolated hypoplastic left heart syndrome. HGG Adv 2023;4:100232. [Crossref] [PubMed]
- Lashkarinia SS, Chan WX, Motakis E, et al. Myocardial Biomechanics and the Consequent Differentially Expressed Genes of the Left Atrial Ligation Chick Embryonic Model of Hypoplastic Left Heart Syndrome. Ann Biomed Eng 2023;51:1063-78. [Crossref] [PubMed]
- Ye S, Wang C, Xu Z, et al. Impaired Human Cardiac Cell Development due to NOTCH1 Deficiency. Circ Res 2023;132:187-204. [Crossref] [PubMed]
- Huang M, Akerberg AA, Zhang X, et al. Intrinsic myocardial defects underlie an Rbfox-deficient zebrafish model of hypoplastic left heart syndrome. Nat Commun 2022;13:5877. [Crossref] [PubMed]
- Wang YJ, Zhang X, Lam CK, et al. Systems analysis of de novo mutations in congenital heart diseases identified a protein network in the hypoplastic left heart syndrome. Cell Syst 2022;13:895-910.e4. [Crossref] [PubMed]
- Hill MC, Kadow ZA, Long H, et al. Integrated multi-omic characterization of congenital heart disease. Nature 2022;608:181-91. [Crossref] [PubMed]
- Garcia AM, Toni LS, Miyano CA, et al. Cardiac Transcriptome Remodeling and Impaired Bioenergetics in Single-Ventricle Congenital Heart Disease. JACC Basic Transl Sci 2023;8:258-79. [Crossref] [PubMed]
- Birker K, Ge S, Kirkland NJ, et al. Mitochondrial MICOS complex genes, implicated in hypoplastic left heart syndrome, maintain cardiac contractility and actomyosin integrity. Elife 2023;12:e83385. [Crossref] [PubMed]
- Gabriel GC, Yagi H, Xu X, et al. Novel Insights into the Etiology, Genetics, and Embryology of Hypoplastic Left Heart Syndrome. World J Pediatr Congenit Heart Surg 2022;13:565-70. [Crossref] [PubMed]
- Tripathi D, Reddy S. iPSC model of congenital heart disease predicts disease outcome. Cell Stem Cell 2022;29:659-60. [Crossref] [PubMed]


