
High expression of myeloperoxidase may be related to the occurrence of urethral fistula in hypospadias patients after tubularized-incised plate urethroplasty repair
Highlight box
Key findings
• High expression of myeloperoxidase may be closely related to the occurrence of urethral fistula in hypospadias after tubularized incised plate urethroplasty repair.
What is known and what is new?
• Surgical treatment is currently the only treatment for hypospadias, but the incidence of postoperative complications is still high, among which urethral fistula is the most common one.
• The expression of myeloperoxidase in foreskin of hypospadias with postoperative complications, was significantly higher than that of the foreskin tissue of the uncomplicated group.
What is the implication, and what should change now?
• If myeloperoxidase inhibitors are used before surgery in children with high myeloperoxidase expression, would the incidence of urethral fistula be reduced? It is also our next research direction.
Introduction
Hypospadias is one of the most common congenital malformations of male genitalia. It is characterized by a ventrally ectopic urethral meatus, a ventral penile curvature, and a dorsally hooded foreskin. Its prevalence is about 1/200–1/300, and a study indicate that the incidence in Asia is about (0.9–69)/10,000, but in North America, it comes to (6–129.8)/10,000 (1). In recent years, with the continuous advancement of social industrialization, environmental pollution, the incidence of hypospadias has been increasing (2,3).
At present, the primary objectives of hypospadias repair are the reconstruction of the penile urethra, the transfer of the urethral meatus to the top of the glans, and the correction of the potential penile curvature (4). If the urethral opening is only slightly deviated from the tip of the glans, and there is no urethral stricture, normal urinary function, absence of penile curvature, and the patient has no particular aesthetic concerns, surgery may not be necessary (5). Tubularized incised plate (TIP) urethroplasty repair is one of the most commonly employed surgical techniques, predominantly used for the repair of distal and intermediate type hypospadias (6,7). Although TIP surgery technique is quite mature at present and the operation is gradually standardized, there is still a high incidence of postoperative complications, and urethral fistula is one of the most common complications after surgery (6). The occurrence of urethral fistula often means that a second or more operation is required for repair, which brings physical and psychological impact to the child and economic losses to the family.
Recently, the research on the factors of postoperative urethral fistula mainly focuses on the clinical aspect, and there are few studies on histological reasons. Therefore, this study intends to provide different research perspectives for the study of postoperative complications of hypospadias by exploring the histological risk factors of urethral fistula after hypospadias. It can provide a theoretical basis for preoperative intervention and risk assessment in children with hypospadias. We present this article in accordance with the STARD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-519/rc).
Methods
Tissues
The foreskin tissue of 21 children with hypospadias who underwent TIP surgery for the first time in the Department of Pediatric Urology, Shandong Provincial Hospital Affiliated to Shandong First Medical University from June 2018 to June 2020 was used (there were 118 surgical patients in Shandong Provincial Hospital Affiliated to Shandong First Medical University during the period, and 9 patients had urethral fistula after surgery). Hypospadias is classified based on the location of the urethral opening into distal, intermediate and proximal types. The distal type is characterized by the urethral opening situated beneath the glans or at the coronal sulcus, while the intermediate type features the urethral opening located along the mid-portion of the penile shaft. The patients were followed up for 6 months to determine whether postoperative urethral fistula was developed. Of the 21 patients, 9 had urethral fistula after surgery, and the remaining 12 had no complications. There were 12 cases of distal type (postoperative urethral fistula in 4 cases) and 9 cases of intermediate type (postoperative urethral fistula in 5 cases). Collected specimens were stored in a −80 ℃ freezer and liquid nitrogen. Three foreskin tissues of urethral fistula group and 3 of uncomplicated group (which chosen by random selection) were analyzed by proteomics, and followed by immunohistochemistry, immunofluorescence and western blot for 21 samples. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Shandong Provincial Hospital (date 2022-05-10/No. 2022-202) and individual consent for this retrospective analysis was waived due to the retrospective nature.
Label-free quantification
The initial analysis involved six cases of in-solution digestion samples (Trypsin, 12-hour digestion time), which were processed and analyzed through nanoflow liquid chromatography-tandem mass spectrometry (nanoLC-MS/MS). Samples were resuspended with 30 µL solvent C respectively [C: water with 0.1% formic acid; D: acetonitrile (ACN) with 0.1% formic acid], separated by nanoLC and analyzed by on-line electrospray tandem mass spectrometry. The experiments were performed on an Easy-nLC 1000 system (Thermo Fisher Scientific, Waltham, MA, USA) connected to a Q-Exactive mass spectrometer (Thermo Fisher Scientific) equipped with an online nano-electrospray ion source; 10 µL peptide sample was loaded onto the trap column (Thermo Scientific Acclaim PepMap C18, 100 µm × 2 cm), with a flow of 10 µL/min for 3 min and subsequently separated on the analytical column (Acclaim PepMap C18, 75 µm × 15 cm) with a linear gradient, from 3% D to 32% D in 60 min. The column was re-equilibrated at initial conditions for 10 min. The column flow rate was maintained at 300 nL/min. The electrospray voltage of 2 kV versus the inlet of the mass spectrometer was used. The mass spectrometer was run under data dependent acquisition mode, and automatically switched under MS and MS/MS mode. MS1 mass resolution was set as 35 K with m/z 350–1,550 and MS/MS resolution was set as 17.5 K under higher-energy collisional dissociation (HCD) mode. The dynamic exclusion time was set as 20 seconds.
Vocalno plot
The volcano plot, a type of scatter-plot utilized for rapid identification of changes in large datasets comprising replicate data, displays significance on the y-axis and fold-change on the x-axis, respectively.
Hierarchical Cluster Analysis (HCA)
HCA is an algorithmic approach to find discrete groups with varying degrees of (dis)similarity in a data set represented by a (dis)similarity matrix.
Gene Ontology (GO) analysis
Blast2GO version 4 was used for functional annotation. Whole protein sequence database was analyzed by BlastP using whole database and mapped, annotated with GO database. Statistically altered functions of different expressed proteins was calculated by Fisher’s exact test in BLAST2GO.
String analysis
The protein-protein interaction network was analyzed using STRING v10 (http://string-db.org/).
DAVID functional annotation tool
For functional characterization, differentially expressed proteins were analyzed through the DAVID functional annotation tool (https://david.ncifcrf.gov/).
Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis
Pathway analysis was processed by KOBAS (http://kobas.cbi.pku.edu.cn/). Pathways with P value <0.05 were recognized as significant changed.
Tissue micro array slide
In order to ensure the consistency of tissue staining, we made the tissues of 21 patients to tissue micro array slide. Recipient paraffin block was chosen with appropriate core size (3 mm in diameter) and corresponding puncher. The target area was taken with a puncher from the original tissue block (donor block) based on hematoxylin and eosin (H&E) result. The core of the recipient block was injected according to the required sequence. A tissue chip embedding instrument was used to repeatedly embed the donor tissue column and the acceptor wax block to ensure complete integration, forming a tissue chip wax block. The modified tissue chip wax block was sliced on the paraffin slicer, with a slice thickness of 4 µm. The slice was floated on the spreader’s 40 ℃ warm water to spread the tissue flat, removed with a slide, and placed into a 60 ℃ oven for baking. After baking, the slide was removed and stored at room temperature.
Immunohistochemistry analysis
The experimental procedure commenced with tissue section preparation. Following dewaxing and hydration steps, heat-induced epitope retrieval was performed using 1 mM ethylenediaminetetraacetic acid (EDTA) (pH 9.0) in a microwave oven. Subsequent steps included endogenous peroxidase blocking with 3% H2O2 (30 min), non-specific binding site blocking with goat serum (30 min), and overnight incubation at 4 ℃ with primary antibodies anti-myeloperoxidase (MPO) (1:100 dilution, 10624-2-AP, Proteintech, Rosemont, IL, USA). Immunodetection was achieved using peroxidase-conjugated secondary antibodies and diaminobenzidine (DAB; Zhongshan, China), followed by hematoxylin counterstaining and mounting. Negative controls were established through primary antibody omission.
Western blot analysis
To determine MPO level, proteins were extracted from the tissues by suspension in radioimmunoprecipitation assay (RIPA) buffer. The experimental protocol involved initial sample centrifugation (12,000 rpm, 4 ℃, 38 min) to obtain supernatant fractions. Protein concentrations were determined through Bradford protein assay or bicinchoninic acid (BCA) method (Sigma, St. Louis, MO, USA). For western blot analysis, 40 µg protein samples were resolved on 8–12% gradient bis-Tris gels and electrotransferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were probed with specific primary antibodies: rabbit anti-MPO (1:1,000) and mouse anti-actin (Proteintech, Rosemont, IL, USA), followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000; ZsBio, China). Protein bands were visualized through enhanced chemiluminescence detection (LAS-4000).
Immunofluorescence
Tissues were fixation, dehydration and embedding. Then, the tissue sections were made. After dewaxing and hydration, it was incubated in 0.3% Triton at room temperature for 20 min, followed by a wash in 0.01 M phosphate-buffered saline (PBS; 5 min ×3). Next, 30 µL/sample goat serum blocking solution was added at room temperature for 60 min, followed by a 1:100 dilution of primary antibody at 30 µL/sample (diluted in goat serum). The cells were placed in a wet box at 4 ℃ overnight and then washed in 0.01 M PBS (5 min ×3). On the next day, the addition of 1:100 fluorescent secondary antibody at 30 µL/sample (10% skim milk powder) at room temperature and in the dark for 60 min, followed by 4',6-diamidino-2-phenylindole (DAPI) was added for 10 min. Subsequently, the cells underwent three 5-minute washing cycles in 0.01 M PBS, followed by mounting with an anti-fluorescence quenching medium.
Statistical analysis
The data were statistically analyzed using Poisson distribution, Student’s t-test, the χ2 test, or Fisher’s exact test using SPSS version 19.0 software (IBM SPSS, Armonk, NY, USA). A P value <0.05 was considered significant. Tandem mass spectra were processed by PEAKS Studio version 8.0 (Bioinfor Inc., Sacramento, CA, USA). Different expressed proteins were filtered if their fold change were over 2-fold and statistical P value below 0.01 (significant >20).
Results
There were differences in protein expression in the foreskin tissue between the urethral fistula group and the uncomplicated group
The results of label-free mass spectrometry showed that compared with the foreskin tissue of the uncomplicated group, there were 32 differentially expressed proteins in the foreskin of the urethral fistula group, among which 28 proteins including MPO, were highly expressed, and 4 proteins were expressed lowly (Figure 1). The results were statistically different (P<0.05).

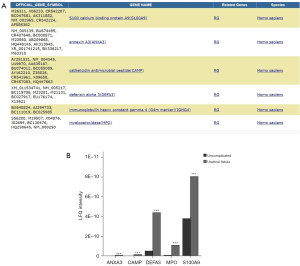
A total of 5 differential proteins were found to be associated with acute inflammatory response
DAVID protein function classification analysis was performed on the screened differential proteins (Figure 2), and the results showed that 5 proteins were involved in acute inflammatory response. These differential proteins are highly expressed, including MPO and its related proteins, such as CAMP, ANXA3, DEFA3, S100A9 (P<0.01, fold change >2).
Interaction analysis between differential proteins
We used STRING to analyze the interrelationships among 32 differentially expressed proteins and found that there were interrelationships among 19 differentially expressed proteins including MPO. A protein interaction network is formed between them, and MPO and related proteins are at the core of the network (Figure 3).

Enrichment analysis of differential proteins
GO enrichment analysis found that there were differences in cell biological behavior, including biological processes, molecular functions, and cellular components. KEGG enrichment identified interleukin-17 (IL-17) and cytokine-mediated inflammatory pathways, which involved MPO (Figure 4).

The expression of MPO in the foreskin tissue of the urethral fistula group was significantly higher than that of the uncomplicated group
To further verify the expression of MPO, we performed immunohistochemistry, immunofluorescence and western blotting (Figure 5). It was verified that the expression of MPO, MPO-positive cells and the rate of MPO-positive cells in the foreskin tissue of the urethral fistula group was significantly higher than that of the foreskin tissue of the uncomplicated group (P<0.05).

The more the percentage of MPO positive cells, the greater the possibility of urethral fistula, which may be a risk factor for urethral fistula after tip operation
We analyzed the results of immunohistochemistry, counted the number of positive cells and the total number of cells under the 400× visual field, then we calculated the percentage of positive cells and the results are shown in Table 1. The difference in the number of positive cells and the percentage of positive cells between the urethral fistula group and the uncomplicated group was statistically significant (P<0.05). Univariate logistic regression was performed on the percentage of positive cells (Figure 6 and Table 2), suggesting that the higher percentage of MPO positive cells was a risk factor of urethral fistula after TIP operation (P<0.05). A critical value of 37.42% was obtained from the receiver operating characteristic (ROC) curve (Figure 6) [area under the curve (AUC) value =0.938, P=0.001, Youden index =0.75]. If the percentage of positive cells is more than 37.42%, the possibility of postoperative urethral fistula is higher.
Table 1
| Variables | Urethral fistula | Uncomplicated | P value |
|---|---|---|---|
| Number of cases | 9 | 12 | – |
| Total number of cells | 126.33±32 | 138.75±38.20 | – |
| Number of MPO positive cells | 79.67±32.44 | 30.83±16.28 | <0.001 |
| Percentage of MPO positive cells | 62.58±15.41 | 25.46±15.99 | <0.001 |
Data are presented as mean ± standard deviation. MPO, myeloperoxidase.

Table 2
| Variable | β | SE | Wald | OR | 95% CI | P value |
|---|---|---|---|---|---|---|
| Percentage of MPO positive cells | 0.128 | 0.063 | 44.82 | 1.148 | 1.015–1.299 | 0.028 |
CI, confidence interval; MPO, myeloperoxidase; OR, odds ratio; SE, standard error; TIP, tubularized incised plate.
Discussion
Urethral fistula is a common complication after hypospadias surgery. For pediatric urology surgeons, the topic of post-operative complications after hypospadias repair and their associated risk factors has always been an interesting subject matter to be studied and discussed (8). The research on postoperative complications of hypospadias has always been the focus of research by pediatric urologists (9,10). At present, most researches on the occurrence of postoperative urethral fistula focus on clinical exploration. And, urethral plate width has been studied in a considerable part (11,12). Some studies have pointed out that urethral plate width may increase the risk of postoperative urethral fistula (13), however, Snodgrass and colleagues in previous studies showed that urethral plate width had no relationship with the prognosis of TIP surgery (14,15). Therefore, currently, there is no definite conclusion. And less research has been done in histology.
We analyzed the differences in protein expression between the urethral fistula group and the no urethral fistula group by proteomics, and found 32 differentially expressed proteins, 28 of which were highly expressed and 4 were under-expressed. Through the method of bioinformatics, we conducted differential protein function analysis and found that IL-17-mediated signaling pathway and cytokine-mediated immune response pathway played an important role. Among them, the inflammatory response mediated by IL-17 plays an important role in the process of injury, physiological stress and infection (16). We functionally classified 32 differentially expressed proteins through the DAVID database and found 5 differentially expressed proteins related to inflammation, of which MPO was most significantly different between the two groups. At the same time, as an inflammatory factor, MPO is closely related to the immune pathway mediated by IL-17 (17,18). Therefore, we conducted further studies with MPO as our target protein.
MPO is a secreted protein that mainly exists in neutrophils and monocytes. Immune stimulation can lead to the activation and aggregation of neutrophils, and release a large amount of MPO at the aggregation site, which in turn initiates oxidative stress and cause the occurrence and development of inflammation-related diseases (19). The biological function MPO involves its release from immune cells, primarily neutrophils and monocytes, during inflammatory responses. Upon stimulation by inflammatory mediators, these cells undergo cytoskeletal remodeling, migrate through the endothelium of capillaries in response to cytokines, and infiltrate inflamed tissues, where they degranulate and release MPO to exert its activity (19,20). After released to the inflammatory site, MPO catalyzes the production of H2O2 and NH2CI, which play a cytotoxic effect. H2O2 and NH2CI are substances with strong biological activity and strong antimicrobial effects. Therefore, MPO plays an important role in the body’s resistance to pathogen invasion and the promotion of tissue inflammatory response. However, the inflammatory response is a double-edged sword. Apart from resisting the invasion of external pathogens, the inflammatory response also damages normal tissues (19,21,22). During the whole operation, the inflammatory reaction is involved in the ischemia-reperfusion of the tissue during the operation and the wound healing after the operation. In these places, inflammatory reactions often bring some negative results.
So far, there are no studies investigating whether MPO expression in foreskin samples is a risk factor for the development of urethral fistula after TIP surgery, and this study is the first to report the effect of MPO on the occurrence of urethral fistula after TIP surgery, that is, MPO is highly expressed in the foreskin of the urethral fistula group, which is a histological risk factor for urethral fistula after hypospadias. We showed that if the percentage of MPO-positive cells in the tissue is greater than 37.42%, the risk of postoperative urethral fistula is significantly increased, and the percentage of MPO-positive cells can be used as a clinical indicator to predict the prognosis after TIP hypospadias. Combined with the analysis of the biological role of MPO, it can be speculated that the occurrence of urethral fistula after TIP surgery is likely to be closely related to the immune response which involves in MPO. Maybe it is this strong immune response that causes tissue damage. The research on MPO inhibitors is currently a hot topic (23,24). If MPO inhibitors are used before surgery in children with high MPO expression, would the incidence of urethral fistula be reduced? It is also our next research direction. The limitation of this article is the need to expand the sample size to further validate the impact of MPO on the occurrence of urethral fistula after hypospadias surgery and to verify its underlying mechanism.
Conclusions
In this study, firstly, we found differences in the protein expression of preoperative foreskin tissue between the urethral fistula group and the uncomplicated group; secondly, MPO was highly expressed in the urethral fistula group. Last but not least, the high expression of MPO is a risk factor for the occurrence of urethral fistula after TIP surgery, and it provides a theoretical basis for the prevention of urethral fistula.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-519/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-519/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2024-519/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2024-519/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Ethics Committee of the Shandong Provincial Hospital (date 2022-05-10/No. 2022-202) and individual consent for this retrospective analysis was waived due to the retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van der Horst HJ, de Wall LL. Hypospadias, all there is to know. Eur J Pediatr 2017;176:435-41. [Crossref] [PubMed]
- Springer A, van den Heijkant M, Baumann S. Worldwide prevalence of hypospadias. J Pediatr Urol 2016;12:152.e1-7. [Crossref] [PubMed]
- Doğan G, İpek H. The evolution of hypospadias publications: A bibliometric approach. Rev Int Androl 2021;19:224-33. [Crossref] [PubMed]
- Tatanis V, Katsakiori P, Spinos T, et al. Anterior and Mid-Penile Hypospadias Repair with TIP Technique-Is It Possible with 20-Hour Catheterization? Diseases 2024;12:279. [Crossref] [PubMed]
- Bohane E, Murphy M, Chierigo F, et al. Long term outcomes from uncorrected hypospadias: a scoping review. Actas Urol Esp (Engl Ed) 2025;49:1-10. [Crossref] [PubMed]
- Snodgrass W, Bush N. TIP hypospadias repair: A pediatric urology indicator operation. J Pediatr Urol 2016;12:11-8. [Crossref] [PubMed]
- Hamid R, Baba AA. Comparison of outcome of TIP urethroplasty with or without Buck's Fascia repair. BMC Urol 2024;24:133. [Crossref] [PubMed]
- Duarsa GWK, Tirtayasa PMW, Daryanto B, et al. Risk factors for urethrocutaneous fistula following hypospadias repair surgery in Indonesia. J Pediatr Urol 2020;16:317.e1-6. [Crossref] [PubMed]
- Dokter EM, van der Zanden LF, Laumer SJ, et al. Development of a prediction model for postoperative complications after primary hypospadias correction. J Pediatr Surg 2020;55:2209-15. [Crossref] [PubMed]
- Ji F, Tang H, Wu C, et al. Predictive Value of C-Reactive Protein for Early Postoperative Complications in Children After Hypospadias Surgery. Front Pediatr 2021;9:690863. [Crossref] [PubMed]
- Galal M, Taha DE, Elabden KZ, et al. The Effect of Pre-Incision Urethral Plate Width and Glanular Width on the Outcome of Tubularized Incised Urethral plate repair surgery in Distal Penile Hypospadias, A prospective study. Urol J 2021;19:50-5. [PubMed]
- Chukwubuike KE, Obianyo NEN, Ekenze SO, et al. Assessment of the effect of urethral plate width on outcome of hypospadias repair. J Pediatr Urol 2019;15:627.e1-6. [Crossref] [PubMed]
- Aboutaleb H. Role of the urethral plate characters in the success of tubularized incised plate urethroplasty. Indian J Plast Surg 2014;47:227-31. [PubMed]
- Bush NC, Snodgrass W. Pre-incision urethral plate width does not impact short-term Tubularized Incised Plate urethroplasty outcomes. J Pediatr Urol 2017;13:625.e1-6. [Crossref] [PubMed]
- Nguyen MT, Snodgrass WT, Zaontz MR. Effect of urethral plate characteristics on tubularized incised plate urethroplasty. J Urol 2004;171:1260-2; discussion 1262. [Crossref] [PubMed]
- McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019;50:892-906. [Crossref] [PubMed]
- Droeser RA, Mechera R, Däster S, et al. MPO density in primary cancer biopsies of ovarian carcinoma enhances the indicative value of IL-17 for chemosensitivity. BMC Cancer 2016;16:639. [Crossref] [PubMed]
- Lee HC, Liao CC, Day YJ, et al. IL-17 deficiency attenuates acetaminophen-induced hepatotoxicity in mice. Toxicol Lett 2018;292:20-30. [Crossref] [PubMed]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol 2005;77:598-625. [Crossref] [PubMed]
- Ndrepepa G. Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin Chim Acta 2019;493:36-51. [Crossref] [PubMed]
- Coskun A, Arikan DC, Coban YK, et al. The effect of ovariectomy on the skin flap viability and myeloperoxidase levels. Bratisl Lek Listy 2014;115:766-70. [Crossref] [PubMed]
- Ramachandra CJA, Ja KPMM, Chua J, et al. Myeloperoxidase As a Multifaceted Target for Cardiovascular Protection. Antioxid Redox Signal 2020;32:1135-49. [Crossref] [PubMed]
- Galijasevic S. The development of myeloperoxidase inhibitors. Bioorg Med Chem Lett 2019;29:1-7. [Crossref] [PubMed]
- Chaikijurajai T, Tang WHW. Myeloperoxidase: a potential therapeutic target for coronary artery disease. Expert Opin Ther Targets 2020;24:695-705. [Crossref] [PubMed]


