
Prenatal diagnosis of Apert syndrome with continuation of pregnancy—a report of two cases
Highlight box
Key findings
• This study presents two cases of prenatal diagnosis of Apert syndrome, a rare genetic condition characterized by craniosynostosis, midface hypoplasia, and symmetrical syndactyly.
• In both cases, two-dimensional (2D) and three-dimensional (3D) ultrasound findings were suggestive of the syndrome and led to confirmation through fetal exome sequencing.
• One case resulted in a live birth with multidisciplinary care, while the other evolved to intrauterine fetal demise.
What is known and what is new?
• Apert syndrome is a rare but significant condition, characterized by a triad of multisuture craniosynostosis, midface hypoplasia, and symmetric syndactyly of the hands and feet. Early prenatal diagnosis is challenging but essential for appropriate counseling and perinatal planning.
• The report highlights the variability in clinical outcomes, ranging from live birth to stillbirth, even with timely diagnosis. This manuscript adds new evidence on the utility of detailed ultrasound imaging—especially 3D modalities—and fetal exome sequencing in achieving a definitive prenatal diagnosis for Apert syndrome.
What is the implication, and what should change now?
• The findings support the integration of advanced imaging techniques and molecular testing in the diagnostic workup of fetuses with craniofacial abnormalities.
• Increased awareness of prenatal imaging features of Apert syndrome can lead to earlier diagnosis, more accurate counseling, and optimized perinatal care.
• Genetic counseling should be offered even when no family history is present, as most cases result from de novo mutations.
Introduction
Apert syndrome is a rare and severe autosomal dominant disease characterized by a triad of features: multisuture craniosynostosis, midface hypoplasia, and symmetrical syndactyly of the hands (1). It was first described in 1894 by Wheaton, but Dr. Eugene Charles Apert, in 1906, published a summary of nine cases, pioneering the documentation of the syndrome (2). Its prevalence is estimated at 15 cases per million live births and accounts for 4.5% of all patients with craniosynostosis syndromes (3). Individuals with Apert syndrome may exhibit numerous important clinical characteristics, such as feeding difficulties, dental abnormalities, hearing loss, hyperhidrosis, and progressive synostosis of various bones (skull, hands, feet, carpal, tarsal, and cervical vertebrae). Airway obstruction at various levels due to nasal passage narrowing and/or tracheal anomalies is common. Non-progressive ventriculomegaly is present in most individuals, with a small subset developing hydrocephalus. Most individuals with Apert syndrome have normal intelligence or mild intellectual disability, although moderate to severe intellectual disability has been reported in some cases (4). A minority have structural heart abnormalities, gastrointestinal malformations, and genitourinary tract anomalies (4).
Prenatal diagnosis of Apert syndrome using imaging studies is generally achieved in the third trimester of pregnancy when cranial abnormalities become apparent. However, some studies have demonstrated diagnosis as early as the second trimester (5). Confirmation is achieved by identifying a heterozygous pathogenic variant in the fibroblast growth factor receptor 2 (FGFR2) gene through molecular genetic testing of amniotic fluid samples (6).
This study aims to report two cases of prenatal diagnosis of Apert syndrome and pregnancy outcomes. Ultrasound examinations were performed using a Voluson E6 system (GE Healthcare, Zipf, Austria) with a real-time abdominal broad-band (RAB) 4–8L transducer. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-90/rc).
Case presentation
Case 1
A 32-year-old female, secundiparous with previous cesarean section, with no complications in her previous full-term pregnancy, body mass index (BMI) of 26.4 kg/m2, and no pre-existing comorbidities. There was no family history of craniosynostosis, congenital anomalies, or genetic syndromes. The first trimester scan (12 weeks and 4 days) showed a low risk for chromosomal abnormalities (Figure 1).
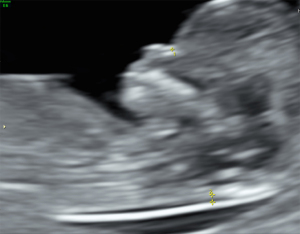
During the second trimester scan (23 weeks and 3 days), fetal anatomy appeared normal. However, the number of fingers could not be assessed due to the persistent closed position of the hands.
In the third trimester (29 weeks and 6 days), an obstetric ultrasound identified brachycephaly, a prominent nasal bone, bilateral syndactyly of the hands, and the left foot. The mother was advised about the possibility of Apert syndrome and Pfeiffer syndrome as differential diagnoses. Fetal exome sequencing, fetal karyotype, fetal echocardiography, and a follow-up ultrasound in 3 weeks were requested. The fetal exome sequencing at 31 weeks and 5 days collected by amniocentesis detected a pathogenic variant [c.755C>G p.(Ser252Trp)] in heterozygosity in the FGFR2 gene, confirming Apert syndrome. Singleton exome sequencing was performed on amniotic fluid samples. Parental samples were not available.
A follow-up ultrasound at 32 weeks and 3 days showed a prominent forehead and persistently closed hands. At 35 weeks, another scan confirmed these findings, along with a potential duplication of the left kidney’s renal pelvis (Figure 2).
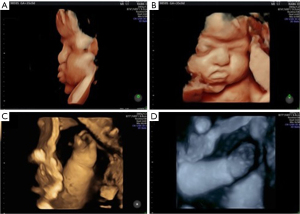
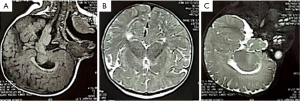
The pregnancy progressed without clinical complications. An elective cesarean section was performed at 38 weeks and 4 days, delivering a female newborn weighing 3,120 grams, with Apgar scores of 8 and 9 at the 1st and 5th minute, respectively. The newborn was admitted to intensive care unit due to respiratory distress for 9 days, then transferred to a ward for 3 days before being discharged. The postnatal X-rays of the hand confirmed the syndactyly (Figure 3). Postnatal brain magnetic resonance imaging (MRI) confirmed the brachycephaly (Figure 4). The infant has since undergone corrective surgeries and continues multidisciplinary follow-up.
Case 2
A 33-year-old white female, secundiparous with previous cesarean section, with a BMI of 21.2 kg/m2, initiated prenatal care timely, took folic acid before conception, and underwent a first trimester scan at 12 weeks and 1 day, showing low risk for chromosomal abnormalities. Family history was unremarkable for genetic conditions or craniofacial anomalies.
At 22 weeks and 3 days, a second trimester scan revealed a prominent forehead, moderate ventriculomegaly, and hypertelorism. The upper limbs showed bilateral finger overlap and subjectively reduced thumbs and halluces. The couple was advised to proceed with fetal karyotyping, fetal exome sequencing, fetal echocardiography, and serial ultrasounds every 3–4 weeks.
At 22 weeks and 4 days, a fetal exome was collected by amniocentesis, detecting the pathogenic variant [c.755C>G p.(Ser252Trp)] in heterozygosity in the FGFR2 gene, associated with Apert syndrome. The analysis was performed as singleton exome sequencing.
A new ultrasound examination at 26 weeks and 4 days showed the same pattern as the ultrasound examination performed at 22 weeks and 2 days. By 29 weeks and 4 days, cerebral abnormalities worsened, with mild left and severe right ventriculomegaly and bilateral hand syndactyly. At 32 weeks and 5 days and 35 weeks and 4 days, it was possible to confirm frontal prominence (craniosynostosis) and syndactyly, in addition to the findings already characterized in previous ultrasound examinations (Figures 5,6).
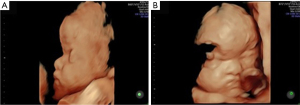
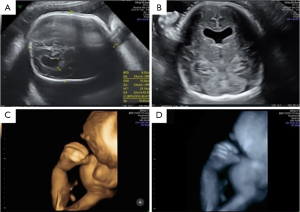
At 38 weeks, intrauterine fetal demise was confirmed, and a cesarean section was performed. The stillbirth weighed 2,575 grams and measured 44.4 cm.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patients and their families for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Prenatal diagnosis of Apert syndrome begins with specific and nonspecific ultrasound findings (7). As in postnatal clinical diagnosis, prenatal diagnosis depends on detecting the same characteristic triad: syndactyly of the hands and feet, abnormal skull shape, and midface hypoplasia. Regarding the extremities, syndactyly of the hands and feet can sometimes be visualized by mid-second trimester. However, there is concern that, in some cases, the syndrome may not be detected until the third trimester due to the difficulty in diagnosing cranial abnormalities early (8). Thus, a synthesis of ultrasound findings can suggest an increased risk of the fetus having the syndrome. The presence of general features such as markedly echogenic forehead, brachycephaly, acrocephaly, and prominent parietal lobes has already been associated with affected fetuses (9). Supporting the literature, case 1 demonstrated that until the 23rd week, no suggestive alterations of craniosynostosis were observed. Although it was not possible to confirm syndactyly of the hands, closed finger positioning was noted. From the 29th week, brachycephaly and bilateral syndactyly of the hands and the left foot were confirmed.
Suspicious ultrasound findings warrant genetic investigation. Mutations in FGFR2, located on chromosome 10, are responsible for almost all known cases of Apert Syndrome (6,9). FGFRs regulate cranial suture fusion. It is known that abnormalities in FGF receptor signaling can impair skull growth and midfacial development (10), resulting in the syndrome’s pathognomonic signs. In case 1, fetal exome confirmed the prenatal syndrome’s diagnosis. The mother was informed and counseled about the syndrome’s characteristics and risks for the fetus, as well as the possible need for corrective surgeries within the first year of life.
Most diagnosed cases of Apert Syndrome are sporadic, with a correlation to advanced paternal age (4). Genetic counseling is crucial. In the majority of cases where the mutation arises de novo, as in these two cases, the recurrence risk in future pregnancies is low. However, if the variant is inherited, there is a 50% recurrence risk due to autosomal dominant inheritance. This distinction is essential for accurate reproductive planning (11). Case 1 resulted in a successful delivery, followed by integrated, multidisciplinary treatment for the child. Case 2, however, resulted in intrauterine fetal demise.
In these case reports, 3D ultrasound in the rendering mode was of great importance for the visualisation of fetal craniofacial malformations as well as for a better understanding of the fetal anomalies by the parents. 3D ultrasound has been used in prenatal diagnosis of Apert syndrome to improve understanding of fetal anomalies by parents and medical team (12). Werner et al. (12) described three prenatal diagnoses of Apert syndrome using two-dimensional (2D) and three-dimensional (3D) ultrasound, MRI and 3D physical/virtual reconstruction models. The main 2D/3D ultrasound and MRI findings were craniosynostosis, hypertelorism, low ear implantation, increased renal size and syndactyly of the hands and feet. David et al. (5) described five cases of prenatal diagnosis of Apert syndrome using 2D and 3D ultrasound. 3D ultrasound was used to show parents the extent of abnormalities of the skull, face and extremities. The parents were advised by craniofacial surgeons and geneticists.
Genetic counseling provided to both couples included information about the autosomal dominant inheritance pattern, implications of a de novo mutation, and the low recurrence risk in future pregnancies. Additionally, the importance of early fetal anomaly screening and the option of targeted genetic testing in future pregnancies were discussed.
Conclusions
In conclusion, we presented two cases of prenatal diagnosis of Apert syndrome by 2D and 3D ultrasound with confirmation by exome obtained by amniocentesis. These cases highlight the importance of confirmation of prenatal diagnosis by exome in cases of fetal craniofacial abnormalities.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-90/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-90/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-90/coif). E.A.J. serves as an unpaid editorial board member of Translational Pediatrics from October 2023 to September 2025. T.M.R.D.C.C. and L.R.M.F.S. are employees of Sabin Diagnostic Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patients and their families for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Souayeh N, Marzouk A, Rouis H, et al. Prenatal diagnosis and management of Apert syndrome in a low-middle income country: Case report. Int J Surg Case Rep 2024;122:110134. [Crossref] [PubMed]
- Kaufmann K, Baldinger S, Pratt L. Ultrasound detection of Apert syndrome: a case report and literature review. Am J Perinatol 1997;14:427-30. [Crossref] [PubMed]
- Cohen MM Jr, Kreiborg S. An updated pediatric perspective on the Apert syndrome. Am J Dis Child 1993;147:989-93. [Crossref] [PubMed]
- Wenger TL, Hing AV, Evans KN. Apert Syndrome. 2019 May 30. In: Adam MP, Feldman J, Mirzaa GM, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2024.
- David AL, Turnbull C, Scott R, et al. Diagnosis of Apert syndrome in the second-trimester using 2D and 3D ultrasound. Prenat Diagn 2007;27:629-32. [Crossref] [PubMed]
- Schmid M, Stary S, Blaicher W, et al. Prenatal genetic diagnosis using microarray analysis in fetuses with congenital heart defects. Prenat Diagn 2012;32:376-82. [Crossref] [PubMed]
- Skidmore DL, Pai AP, Toi A, et al. Prenatal diagnosis of Apert syndrome: report of two cases. Prenat Diagn 2003;23:1009-13. [Crossref] [PubMed]
- Filkins K, Russo JF, Boehmer S, et al. Prenatal ultrasonographic and molecular diagnosis of Apert syndrome. Prenat Diagn 1997;17:1081-4. [Crossref] [PubMed]
- Anderson J, Burns HD, Enriquez-Harris P, et al. Apert syndrome mutations in fibroblast growth factor receptor 2 exhibit increased affinity for FGF ligand. Hum Mol Genet 1998;7:1475-83. [Crossref] [PubMed]
- Purushothaman R, Cox TC, Maga AM, Cunningham ML. Sinostose facial de neonatura Fgfr1 (P250R/+) e Fgfr2 (S252W/+) de síndromes de Pfeiffer e Apert. Defeitos de nascimento Res A Clin Mol Teratol. 2011;91:603-9.
- Khan S, Chatra L, Shenai P, et al. Apert syndrome: a case report. Int J Clin Pediatr Dent 2012;5:203-6. [Crossref] [PubMed]
- Werner H, Castro P, Daltro P, et al. Prenatal diagnosis of Apert syndrome using ultrasound, magnetic resonance imaging, and three-dimensional virtual/physical models: three case series and literature review. Childs Nerv Syst 2018;34:1563-71. [Crossref] [PubMed]



