Indoor/outdoor not-voluptuary-habit pollution and sleep-disordered breathing in children: a systematic review
Introduction
Sleep-disordered breathing (SDB) is a general term for several chronic conditions in which partial or complete cessation of breathing occurs many times throughout the night. Symptoms may include snoring, pauses in breathing and disturbed sleep. Obstructive sleep apnea syndrome (OSAS), which is by far the most common form of SDB, is characterized by episodes of complete or partial upper airway obstruction during sleep (1).
Children with OSAS are increasingly recognized as an important group of patients (2). In childhood, OSAS is most frequently due to tonsil and adenoid hypertrophy. However, obesity and anatomic alterations of the upper airways play a role (3). In childhood, the prevalence of OSAS is in the range of 1% to 5%, making this a relatively common disease (3,4). Screening and early treatment are recommended for children at high-risk (5,6).
The complications of OSAS are sleep fragmentation, neurocognitive, behavioural (7), cardiovascular (8) and artery hypertension (9,10). Increased levels of inflammation have been found in children with OSAS (11), linking cardiovascular pathologies with secondary oxidative stress and intermittent hypoxia (12).
Low-levels of tobacco smoke exposure have been associated with increased inflammatory biomarkers in children with asthma (13). The secondhand smoke has been associated with SDB (evidence level 3b) (14). SDB is prevalent in asthmatic children and its prevalence increased with increasing asthma severity (15). Air pollutants, such as NO2 and particles from diesel exhausts, have adjunct effects on the pathogenesis of asthma and chronic bronchitis, which increased significantly with an increasing traffic pollution load (16). Early traffic-related air pollution exposure (particulate matter—PM2.5 and black carbon) was related to asthma and allergic diseases in children (17). Allergies have also been associated with airway inflammation and sleep disturbances in children (18).
Urban outdoor air pollution refers to the air pollution experienced by populations living in and around urban areas. Indoor air pollution refers to the pollutants found in indoors. The main cause of indoor air pollution is inefficient fuel combustion from rudimentary technologies. In adults, increases in SDB indices or percentage of sleep time at less than 90% O2 saturation and decreases in sleep efficiency were all associated with increases in short-term variations in PM10 from urban areas (19).
Aims of the review
We hypothesized that environmental air pollution can play a role in childhood SDB. The aim of this review was to find if existing researches warrant the conclusion of an association between indoor and outdoor environmental pollution (not from voluptuary habit) and SDB in children.
Methods
We conducted an electronic search in Medline (with PubMed interface), Scopus and the ISI Web of Science using the keywords “sleep” or “sleep apnea” or “sleep disordered breathing” and “pollution” and “children” in “Title/Abstract/Keywords”, with language restriction (non-English paper) and no date limitation to present. All the articles that responded to the search criteria were systematically reviewed by two authors (Marco Zaffanello and Laura Tenero). Asthma and tobacco smoke’s topics were subsequently excluded because they were not pertinent to the review. The references of the selected articles were also hand-searched to identify other pertinent reports. We examined the strength of the evidence according to the Oxford Centre for Evidence-Based Medicine [2011] and the Centre for Evidence-Based Medicine [2009].
Results
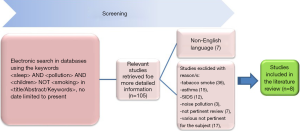
A total of 105 published articles were identified, but 97 of these had to be excluded after an accurate reading of the title, abstract or full text (Figure 1). More specifically, studies were excluded due to: tobacco smoke (n=36), asthma (n=15), SIDS (n=12), noise pollution (n=3), various non-pertinent reviews (n=7), and articles not pertinent to the subject (n=17). Moreover, 7 papers were not in English, the preferred language. In the end, 8 studies were selected for our analysis (Table 1) (20-27).
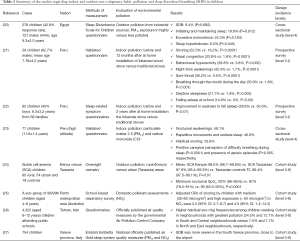
Full table
There were a total of 5,826 enrolled children. Diagnosis of SDB was made subjectively (questionnaires) in six studies (20-23,25,26) and objectively (instrumentation) in two studies, including otherwise healthy (27) or with sickle cell anaemia (24) children, respectively. Five studies quantified the environmental pollution, according to PMs and/or NO2 (20,23,25-27), or considered the possible differences between rural versus urban areas (24) or before/after indoor intervention (21,22).
Interestingly, two studies showed the beneficial effects of using less-polluting smoke stoves over SDB symptoms in children who were using before traditional smoke stoves (21,22). However, the exposure to nitrogen dioxide (NO2) in a domestic environment during winter was significantly associated with snoring in children. In particular, high exposure (NO2 >60 microg/m3) category showed higher OR of snoring (4.5; 95% CI: 1.4–14.3) than media exposure (30–60 microg/m3) category (2.5; 95% CI: 0.7–8.7) (25). Furthermore, significantly higher habitual snoring frequencies was found among children residing in neighbourhoods with great pollution exposure (South 24.5% and Central 12.1%) versus lower pollution exposure (North 7.0% and East 7.7%) (26). Moreover, the geographical variation of SDB in children showed higher severity in the South part of the Varese Italian province, particularly in the Western zone close to the airport, matching the PM10 and NO2 distribution (27). All studies were of 3b evidence levels.
A summary of the results observed is as follows: between highly versus less polluted environments, there were significant differences in snoring (25,26), initiating and maintaining sleep (20-22), sleep hyperhidrosis (20), difficulty breathing and apnea (21,23,27), and, for sickle cell anaemia children, mean and minimal nocturnal saturation (24). These results suggest an involvement (grade C) of environmental (not from voluptuary habits) pollution in the worsening of SDB in children.
Discussion
Exposure to environmental pollutants is advocated to be a major risk factor, with increased morbidity and mortality in humans due to acute and chronic airway inflammation (28). PM2.5 environmental pollution, in particular black carbon (a traffic-related PM2.5 constituent), from proximity to major roadways has been associated with lower lung function in the Boston, USA, area (29). Moreover, from a study conducted at a day-care center in northeastern Seoul, Korea, indoor air pollutants resulting from nearby heavy traffic and a metro station increased the risk of allergy in children (aged 4.4±1.2 years). In addition, toluene from the indoor environment was found to be an aggravating factor. Indeed, symptoms significantly increased by 12.7% (95% CI: −0.01 to 27.1) as indoor levels of toluene increased by 1 ppb (P=0.05) (30). Moreover, long-term exposure to PM10 and NO2 has been associated with cause-specific mortality in the Dutch population (31).
Exposure to smoke impairs ciliary function in pediatric airways. Furthermore, environmental tobacco smoke exposure in children increases the incidence of upper respiratory infections, chronic sinusitis, and chronic otitis media (32). In addition, some studies found a significant association between secondhand smoke and childhood SDB (14,33,34).
Exposure to biomass smoke in rural areas may account for the higher prevalence of snoring and observed apnea by parents and grandparents of students from 20 randomly selected primary schools in urban and rural areas of Turkey. In particular, snoring and the observed apnea were more prevalent among parents and grandparents of students from rural areas [52.6% vs. 46.6%, odds ratio (OR) 1.2; P<0.001] than among those from urban areas (16.2% vs. 10.1%, OR 1.7; P<0.001) (35). Moreover, annual exposure to air pollution was associated with SDB and to a change in blood pressure among 3,762 Taiwan patients. The association between annual air pollution exposure and diastolic blood pressure accounted for high AHI (PM2.5: OR 0.49; P=0.03) and increased BMI (PM2.5: OR 0.52; P=0.04) (36). Unfortunately, the above research has not been conducted on a childhood population.
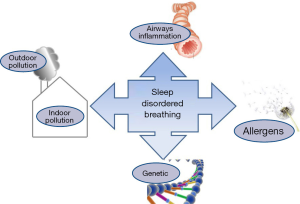
Exposure to indoor and outdoor pollutants may increase the incidence, severity and persistence of SDB in youth. Pathogenic mechanisms can be related to an interaction between genes and various pollutants, which leads to allergies and chronic inflammation of the upper airways (Figure 2). Although there are few studies in the topics, the results encourage further investigations. In particular, four related studies concern indoor pollution sources (21-23,25) and four studies concern outdoor pollution sources (20,24,26,27) of which one was designed for children with sickle cell anaemia (24). Two studies showed reductions in SDB following successful air-quality home-based interventions (21,22). Three studies come from the same research team (21-23); but the places of the studies were not the same, so they evaluated different cohorts. However, one communication reported higher severity of SDB in children living close to the airport (27) and two studies showed higher habitual snoring among children living in place with greater environmental pollution (25,26).
A possible limitation comes from one study: sickle cell anemia children are non-healthy and hypoxemic by definition (24). Moreover, other possible limitation of these studies is that they mainly used questionnaires to screen for SDB in children exposed to environmental pollution, this is less reliable as a real measurement of SDB level (37). Therefore, some research has supported the validity of questionnaires and the convergence of polysomnography and questionnaires for assessing SDB (38,39). It is not easy to conduct studies on air pollution and its impact on health in general and especially, on a complicated, multifactorial disease like SDB.
There are a few medical studies on children living in the most industrialized countries, although the global impact of city traffic and industries on the health of the human population is often an issue. Moreover, exposures assessed among the studies are very heterogeneous, making a systematic review more challenging. That heterogeneity of exposure measurements should be acknowledged.
Conclusions
There is currently some interesting information in the literature concerning SDB in children exposed to indoor/outdoor pollution. In particular, some studies reported significant differences between areas with higher and lower pollutants and the interventions on indoor pollution reduced sleep-disordered breathing in children. Therefore, although the relevance of the argument is high, the number of studies and the interest in the subject seems at this time quite limited.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Alonso-Álvarez ML, Navazo-Egüia AI, Cordero-Guevara JA, et al. Respiratory polygraphy for follow-up of obstructive sleep apnea in children. Sleep Med 2012;13:611-5. [Crossref] [PubMed]
- Jobe AH, Tibboel D. Update in pediatric lung disease 2013. Am J Respir Crit Care Med 2014;189:1031-6. [Crossref] [PubMed]
- Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012;130:e714-55. [Crossref] [PubMed]
- Kaditis AG, Finder J, Alexopoulos EI, et al. Sleep-disordered breathing in 3,680 Greek children. Pediatr Pulmonol 2004;37:499-509. [Crossref] [PubMed]
- Atkinson M. Health CSCatRCoPaC. Sleep, snoring and acute life-threatening events. Arch Dis Child Educ Pract Ed 2010;95:190-3. [Crossref] [PubMed]
- Vitelli O, Miano S, Tabarrini A, et al. Epilepsy and sleep-disordered breathing as false friends: a case report. J Child Neurol 2014;29:NP114-7. [Crossref] [PubMed]
- Hunter SJ, Gozal D, Smith DL, et al. Effect of Sleep-disordered Breathing Severity on Cognitive Performance Measures in a Large Community Cohort of Young School-aged Children. Am J Respir Crit Care Med 2016;194:739-47. [Crossref] [PubMed]
- Tagetti A, Bonafini S, Zaffanello M, et al. Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J Hypertens 2017;35:125-31. [Crossref] [PubMed]
- Narang I, Mathew JL. Childhood obesity and obstructive sleep apnea. J Nutr Metab 2012;2012:134202.
- Blechner M, Williamson AA. Consequences of Obstructive Sleep Apnea in Children. Curr Probl Pediatr Adolesc Health Care 2016;46:19-26. [Crossref] [PubMed]
- Zicari AM, Occasi F, Di Mauro F, et al. Mean Platelet Volume, Vitamin D and C Reactive Protein Levels in Normal Weight Children with Primary Snoring and Obstructive Sleep Apnea Syndrome. PloS One 2016;11:e0152497. [Crossref] [PubMed]
- Loffredo L, Zicari AM, Occasi F, et al. Endothelial dysfunction and oxidative stress in children with sleep disordered breathing: role of NADPH oxidase. Atherosclerosis 2015;240:222-7. [Crossref] [PubMed]
- Gill R, Krishnan S, Dozor AJ. Low-level environmental tobacco smoke exposure and inflammatory biomarkers in children with asthma. J Asthma 2014;51:355-9. [Crossref] [PubMed]
- Jara SM, Benke JR, Lin SY, et al. The association between secondhand smoke and sleep-disordered breathing in children: a systematic review. Laryngoscope 2015;125:241-7. [Crossref] [PubMed]
- Goldstein NA, Aronin C, Kantrowitz B, et al. The prevalence of sleep-disordered breathing in children with asthma and its behavioral effects. Pediatr Pulmonol 2015;50:1128-36. [Crossref] [PubMed]
- Ising H, Lange-Asschenfeldt H, Lieber GF, et al. Respiratory and dermatological diseases in children with long-term exposure to road traffic immissions. Noise Health 2003;5:41-50. [PubMed]
- Bowatte G, Lodge C, Lowe AJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 2015;70:245-56. [Crossref] [PubMed]
- Zicari AM, Occasi F, Cesoni Marcelli A, et al. Habitual snoring in children with previous allergic sensitization. Int J Immunopathol Pharmacol 2013;26:565-70. [Crossref] [PubMed]
- Zanobetti A, Redline S, Schwartz J, et al. Associations of PM10 with sleep and sleep-disordered breathing in adults from seven U.S. urban areas. Am J Respir Crit Care Med 2010;182:819-25. [Crossref] [PubMed]
- Abou-Khadra MK. Association between PM10 exposure and sleep of Egyptian school children. Sleep Breath 2013;17:653-7. [Crossref] [PubMed]
- Castañeda JL, Kheirandish-Gozal L, Gozal D, et al. Effect of reductions in biomass fuel exposure on symptoms of sleep apnea in children living in the peruvian andes: a preliminary field study. Pediatr Pulmonol 2013;48:996-9. [Crossref] [PubMed]
- Accinelli RA, Llanos O, López LM, et al. Adherence to reduced-polluting biomass fuel stoves improves respiratory and sleep symptoms in children. BMC Pediatr 2014;14:12. [Crossref] [PubMed]
- Accinelli RA, Llanos O, Lopez LM, et al. Caregiver perception of sleep-disordered breathing-associated symptoms in children of rural Andean communities above 4000 masl with chronic exposure to biomass fuel. Sleep Med 2015;16:723-8. [Crossref] [PubMed]
- L'Esperance VS, Ekong T, Cox SE, et al. Nocturnal haemoglobin oxygen desaturation in urban and rural East African paediatric cohorts with and without sickle cell anaemia: a cross-sectional study. Arch Dis Child 2016;101:352-5. [Crossref] [PubMed]
- Zhang G, Spickett J, Rumchev K, et al. Snoring in primary school children and domestic environment: a Perth school based study. Respir Res 2004;5:19. [Crossref] [PubMed]
- Kheirandish-Gozal L, Ghalebandi M, Salehi M, et al. Neighbourhood air quality and snoring in school-aged children. Eur Respir J 2014;43:824-32. [Crossref] [PubMed]
- Nosetti LM, Montomoli C, Tentoni S, et al. Is PM10 Air Pollution a Risk Factor for Sleep-Disordered Breathing in Children? A Study in the Province of Varese. Am J Respir Crit Care Med 2016;193:A1213.
- Akopian AN, Fanick ER, Brooks EG. TRP channels and traffic-related environmental pollution-induced pulmonary disease. Semin Immunopathol 2016;38:331-8. [Crossref] [PubMed]
- Rice MB, Rifas-Shiman SL, Litonjua AA, et al. Lifetime Exposure to Ambient Pollution and Lung Function in Children. Am J Respir Crit Care Med 2016;193:881-8. [Crossref] [PubMed]
- Kim EH, Kim S, Lee JH, et al. Indoor air pollution aggravates symptoms of atopic dermatitis in children. PloS One 2015;10:e0119501. [Crossref] [PubMed]
- Fischer PH, Marra M, Ameling CB, et al. Air Pollution and Mortality in Seven Million Adults: The Dutch Environmental Longitudinal Study (DUELS). Environ Health Perspect 2015;123:697-704. [PubMed]
- Wang LF, White DR, Andreoli SM, et al. Cigarette smoke inhibits dynamic ciliary beat frequency in pediatric adenoid explants. Otolaryngol Head Neck Surg 2012;146:659-63. [Crossref] [PubMed]
- Yolton K, Xu Y, Khoury J, et al. Associations between secondhand smoke exposure and sleep patterns in children. Pediatrics 2010;125:e261-8. [Crossref] [PubMed]
- Montgomery-Downs HE, Gozal D. Snore-associated sleep fragmentation in infancy: mental development effects and contribution of secondhand cigarette smoke exposure. Pediatrics 2006;117:e496-502. [Crossref] [PubMed]
- Ekici M, Ekici A, Keles H, et al. Risk factors and correlates of snoring and observed apnea. Sleep Med 2008;9:290-6. [Crossref] [PubMed]
- Liu WT, Lee KY, Lee HC, et al. The association of annual air pollution exposure with blood pressure among patients with sleep-disordered breathing. Sci Total Environ 2016;543:61-6. [Crossref] [PubMed]
- Tan HL, Gozal D, Ramirez HM, et al. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep 2014;37:255-60. [PubMed]
- Montgomery-Downs HE, O'Brien LM, Holbrook CR, et al. Snoring and sleep-disordered breathing in young children: subjective and objective correlates. Sleep 2004;27:87-94. [PubMed]
- Spruyt K, Gozal D. Screening of pediatric sleep-disordered breathing: a proposed unbiased discriminative set of questions using clinical severity scales. Chest 2012;142:1508-15. [Crossref] [PubMed]