
Two novel frameshift variations in the ELF4 gene expand the mutational spectrum of DEX: case report and literature review
Highlight box
Key findings
• We present two pediatric cases of novel frameshift variants in ELF4 gene, expanding the spectrum of deficiency in ELF4, X-linked (DEX). Notably, treatment with glucocorticoid and tumor necrosis factor-α (TNF-α) has demonstrated significant therapeutic effectiveness.
What is known and what is new?
• DEX remains a rare immunological disorder typically characterized by recurrent fever of unknown origin, recurrent oral ulcer, inflammatory bowel disease (IBD)-like symptoms, anemia, arthritis, rash or elevated inflammatory markers. Due to its overlapping features, it is often misdiagnosed as Behçet’s disease or IBD.
• In this report, we present two cases of DEX with gastrointestinal involvement and report two new mutation sites.
What is the implication, and what should change now?
• The prevalence of DEX may be underestimated, particularly in pediatric populations with nonspecific inflammatory symptom. Clinicians should raise awareness of this disease, and consider early genetic testing in pediatrics presenting with similar clinical symptoms to facilitate timely and accurate diagnosis.
Introduction
In recent years, the concept of primary immunodeficiency disease (PID) has been gradually updated to include “human inborn errors of immunity (IEI)”. IEI now encompasses 10 categories: (I) combined immunodeficiency; (II) combined immunodeficiency with syndromic features; (III) predominantly antibody deficiency; (IV) immune dysregulation diseases (diseases of immune dysregulation, DID); (V) congenital defects of phagocytes; (VI) innate immunity and innate immunity deficiency; (VII) autoinflammatory diseases (AIDs); (VIII) complement deficiency; (IX) bone marrow failure; and (X) phenocopies of inborn errors of immunity. Among these, deficiency in ELF4, X-linked (DEX) is a recently identified monogenic autoinflammatory disorder under the DID subgroup of IEI (1,2).
E74-like factor (ELF) subfamily members are part of E26 transformation-specific (ETS) transcription factors, which can regulate immune responses and the development of immune-related cells. This subfamily includes ELF1, ELF2, and ELF4/MEF. Notably, ELF4, located on chromosome Xq26, has been previously reported to be highly expressed in peripheral blood and is present in all major tissues except the brain. Human ELF4 is highly expressed in hematopoietic cells such as natural killer (NK) cells, myeloid cells, T cells, and monocytes, and moderately expressed in the lungs, placenta, thymus, and bone marrow (3). ELF4 plays a critical role in immune responses and signaling, osteogenesis, adipogenesis, cancer, and stem cell quiescence. Functioning as a transcriptional activator, ELF4 exerts its effect through interactions with partner proteins. ELF4 directly activates the tumor suppressors KLF4 and KLF2 to regulate cell cycle arrest in naïve CD8+ T cells (4). ELF4 directly regulates perforin transcription in NK cells (5). ELF4 mutants cause diseases because they cannot effectively drive the expression of anti-inflammatory and antiviral genes (5).
The main clinical features of DEX include male predominance and recurrent inflammation affecting the skin and mucosal tissue. These typically present as skin ulcers or rashes, oral ulcers, inflammatory bowel disease (IBD)-like intestinal inflammation, and perianal ulcers. Joint involvement may occur with or without recurrent fever (6). In rare cases, atypical presentations such as autoimmunity, susceptibility to infection, and cancer are also observed (6). Owing to the scarcity of case reports on DEX worldwide, the clinical spectrum and pathophysiology of DEX remain poorly understood. Misdiagnosed cases, particularly as Behçet’s disease or IBD, are not uncommon. In this report, we describe two novel cases of frameshift variations in two young Chinese boys diagnosed with DEX. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-167/rc).
Case presentation
Case 1
Patient 1, a 7-year-and-4-month-old boy, was referred to our department with a 2-month history of frequent perianal ulcers and recurrent fever of unknown origin. Despite receiving intravenous antibiotics in response to elevated inflammatory markers, his fever persisted. Notably, he began experiencing recurrent oral ulcers at the age of 6 years. At 6 years and 11 months, the patient developed a perianal abscess that required incision and drainage. He was the second child in his family. The other family members were healthy. Physical examination revealed oral and perianal aphthous-like ulcers, while examinations of the other systems were normal. Laboratory examination revealed a significant elevation in inflammatory markers, even though body temperature was within the normal range at the time. Blood tests revealed a C-reactive protein (CRP) level of 35 mg/L, whereas the white blood cell count and erythrocyte sedimentation rate (ESR) were within normal limits. Other tests related to infections, such as tuberculosis, Epstein-Barr virus, were negative. Abdominal, cardiac, and large-vessel ultrasonography of the limb yielded negative results. Immunoglobulin examination revealed slightly increased serum immunoglobulin G (IgG) levels (15.25 g/L; reference range, 5–10.6 g/L) and immunoglobulin A (IgA) levels (1.49 g/L; reference range, 0.34–1.38 g/L). Lymphocyte subpopulation analysis revealed low levels of NK cells, CD3−CD16+CD56+ (2.45%, reference range 11–24%), CD19+ B cells (20.95%, reference range 15–20%), CD3+ T cells (78.2%, reference range, 65–72%), CD4+ T cells (37.80%, reference range, 27–34%), and CD8+ T cells (32.85%, reference range, 23–30%).
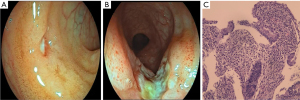
Given the recurrent mucosal ulcers, Behçet’s disease was initially suspected. However, ophthalmologic examination revealed no ocular lesions, and the pathergy test for Behçet’s disease was negative. To further investigate potential gastrointestinal involvement, the patient underwent endoscopy. Gastroscopy revealed superficial gastritis. Colonoscopy revealed multiple ulcers in the terminal ileum and the colon, including the largest ulcer measuring approximately 0.8 cm × 1.4 cm near the ileocecal valve (Figure 1A,1B). Scattered superficial ulcers were also observed from the ascending colon to the sigmoid colon. Capsule endoscopy revealed ulceration in the terminal ileum. Histopathological examination revealed mild chronic active inflammatory cell proliferation, along with scattered eosinophilic and lymphocytic infiltration from the terminal ileum to the sigmoid colon. Active ulcer formation was observed in the terminal ileum; however, no caseating granulomas or crypt abscesses were detected (Figure 1C). Immunohistochemistry for Epstein-Barr virus-encoded RNA (EBER), cytomegalovirus (CMV), and acid-fast staining were negative. Taken together, the patient’s age, endoscopic findings, and pathological findings were more indicative of an IBD-like disorder, possibly with a monogenic etiology. Based on this suspicion, we recommended genetic testing for the patient. The patient was initially treated with total enteral nutrition. Despite good compliance, recurrent ulcers persisted. Finally, the patient was treated with TNF-α blocker adalimumab via subcutaneous injection every 2 weeks. Subsequently, the patient became asymptomatic and received regular doses of adalimumab. Over a 5-month follow-up period under biologic therapy, all inflammatory markers normalized, and colonoscopy revealed complete mucosa healing with no evidence of ulcers. By the time of writing, the patient had been under follow-up for 18 months. Although occasional respiratory tract infections were reported, no recurrent oral ulcers were observed.
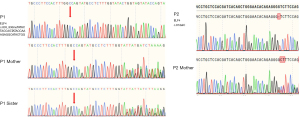
Patient blood samples were collected for analysis before commencing treatment. A comprehensive analysis of lymphocyte subsets revealed a decrease in activated T-regulatory cells (Tregs) compared to age- and sex-matched healthy controls (Table 1). The patient and his family provided informed consent and subsequently underwent whole-exome sequencing (WES). Unfortunately, the patient’s father was unavailable for blood sampling. Next-generation sequencing revealed a hemizygous frameshift mutation in ELF4: chrX-129203626-129203627, exon 8, and c.835_836insTATACTACCAGTATACCAAAGAGGCATACTGG (p.Ala279ValfsTer103). The variant was confirmed by Sanger sequencing and was inherited from the patient’s mother. The same variant was also detected in the patient’s sister (Figure 2).
Table 1
| Immune cell | Denominator | Percentage | Percentage reference range | Absolute numbers (cells/μL) | Absolute numbers reference range (cells/μL) |
|---|---|---|---|---|---|
| Total T cells | Lymphocyte | 68.52 | 43.70–80.50 | 3,036.14† | 711–2,353 |
| CD4+ T cells | Total T cell | 34.96 | 28.06–70.72 | 1,061.58 | 368–1,632 |
| Th1 | CD4+ T cell | 11.36‡ | 12.65–36.24 | 120.56 | 89.88–140.7 |
| Th2 | CD4+ T cell | 64.08† | 22.38–60.19 | 680.23 | 101.7–738.0 |
| Th17 | CD4+ T cell | 10.49 | 7.13–24.53 | 111.35 | 69.11–112.8 |
| Total Treg | CD4+ T cell | 6.82 | 2.483–8.124 | 72.42† | 23.47–58.18 |
| Memory Treg | Total Treg | 22.54‡ | 61.41–93.69 | 16.33 | 15.49–52.43 |
| Activated Treg | Total Treg | 8.09‡ | 32.63–60.38 | 5.86‡ | 11–25.09 |
| Naive Treg | Total Treg | 77.46† | 6.31–38.52 | 56.09† | 2.22–15.09 |
| Total B cells | Lymphocyte | 26.7† | 5.00–18.00 | 1,183.07† | 90.31–350 |
| Memory B | Total B cell | 17.85 | 8.34–34.76 | 211.22† | 15.94–55.64 |
| Naïve B | Total B cell | 36.17‡ | 64.21–87.32 | 427.93† | 79.27–175.1 |
| Transitional B | Total B cell | 12.59† | 0.77–5.99 | 148.91† | 1.14–12.54 |
| Plasmablasts B | Total B cell | 0.10 | 0.08–1.43 | 1.18 | 0.14–2.64 |
| Total monocyte | White blood cell | 4.86 | 4.00–10.00 | 519.98† | 181.6–297.3 |
| Total NK cells | Lymphocyte | 6.07‡ | 7.00–40.00 | 268.78 | 150–1,100 |
| Neutrophil | White blood cell | 51.79 | 40.00–75.00 | 5,539.1 | 1,800–6,300 |
†, increased sub-population; ‡, decreased sub-population. NK, natural killer.
Case 2
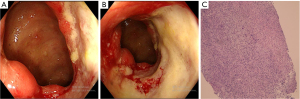
Patient 2 was a 12-year-and-6-month-old boy, presenting with a 1-year history of recurrent oral ulcers and loss of appetite. He also reported paroxysmal abdominal pain, mainly around the umbilicus, without other symptoms. Over the past years, he had lost 5 kg of weight. His previous medical history revealed an anal fistula 6 years prior and an appendectomy 3 years prior. He was a full-term infant with a birth weight of 2,900 g, and his parents were healthy. On admission, physical examination revealed an emaciated appearance, with a height-for-age Z-score of −2.76, body mass index-for-age Z-score of −3.04, deep and large oral ulcers, a soft abdomen, mild tenderness around the umbilicus, and no rebound tenderness in any area. Laboratory tests were as follows: white blood cell count, 10.23×109/L; neutrophil count, 8.20×109/L; hemoglobin, 109 g/L; CRP, 26.48 mg/L; ESR, 36.07 mm/h; and biochemistry, tumor markers, antinuclear antibodies, CMV and Epstein-Barr virus antibodies, T-SPOT, trace elements, fat and water-soluble vitamins and fecal parasites within the normal ranges. Ultrasonographic examination of the large arteries revealed no significant abnormalities. Additionally, ophthalmologic examination revealed no ocular lesions. Magnetic resonance enterography (MRE) demonstrated significant thickening and enhancement of the intestinal wall in the terminal ileum, along with mild exudative changes in the surrounding area. Given the high probability of intestinal lesions, the patient underwent gastroscopy, colonoscopy, and capsule endoscopy. Gastroscopy indicated superficial gastritis. Colonoscopy revealed deformation of the ileocecal region. An ulcer affected approximately half of the ileocecal valve, extending to the ileocecal region and initial part of the ascending colon. The ulcer was difficult to fully visualize and measured approximately 3 cm × 4 cm (Figure 3A,3B). Capsule endoscopy did not reveal any abnormalities in the small intestinal mucosa. Pathological colonoscopy revealed chronic colitis with ulcer formation in the ileocecal valve. The villous structure of the terminal ileum was preserved, with an increase in chronic inflammatory cells, mildly active inflammation, and lymphocyte aggregation. There were 20–40 eosinophils per high-power field (HPF) in the ileocecal valve, and CMV/EBER/acid-fast staining was negative (Figure 3C).
Based on the endoscopic and pathological findings, a high index of suspicion for Behçet disease or IBD was warranted. However, distinguishing between these conditions is challenging due to overlapping clinical and pathological features. Following a multidisciplinary evaluation, gene testing was unanimously recommended. Consequently, whole-exome gene sequencing was carried out following the acquisition of informed consent.
During hospitalization, the patient received corticosteroids and partial enteral nutritional treatment. The corticosteroid dosage was gradually tapered, and oral azathioprine was introduced as maintenance therapy. With more than three months of follow-up, the patient reported no abdominal pain, with normal inflammatory markers, and had gained 5 kilograms in weight.
Genetic testing revealed a frameshift mutation in ELF4 [exon 3: c.87delC (p.S30Lfs*6); XLR]. This mutation leads to the premature occurrence of a stop codon, resulting in a remarkably high degree of pathogenicity. The patient’s mother was identified as a heterozygous carrier (Figure 2).
Ethical consideration
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. Publication of this case report and accompanying images was waived from patient consent according to the Medical Ethics Committee of Children’s Hospital, Zhejiang University School of Medicine (No. 2014-IRB-0386-P-01).
Discussion
The ELF4 gene, located on chromosome Xq26, was originally identified and cloned from megakaryocyte cell lines more than 20 years ago. The gene encodes a protein consisting of 663 amino acids, composed of an N-terminal activation domain, an ETS domain that binds to DNA consensus sequences, a region rich in serine/threonine residues, and a region rich in proline residues (3). In 2021, Tyler et al. first reported variants in the ELF4 gene as the cause of DEX (7). To further investigate the clinical spectrum and genetic characteristics of this rare disorder, we conducted a literature search in PubMed using the keywords “ELF4”, “MEF”, “ELF4 deficiency”, “DEX” or “myeloid ELF-1 like factor” and reviewed all relevant literature published up to January 2025. To our knowledge, only 22 cases have been reported worldwide (2,7-14). In this study, we summarize the clinical and genetic mutation features of the reported patients with DEX, along with our cases, in Table S1.
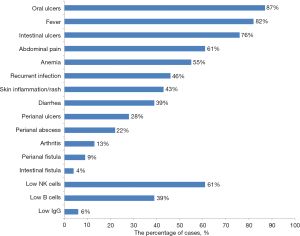
Notably, all reported patients with DEX were male. The age at onset ranged from 20 days to 17 years, with 14 patients (58.3%) developing symptoms before 6 years of age. Only three of the DEX patients had a family history, including patients with recurrent oral ulcers or Behçet’s disease. The predominant clinical manifestations included oral and intestinal ulcers, fever, abdominal pain, and anemia (Figure 4). Two patients developed severe digestive tract complications and required surgical intervention due to intestinal fistula and gastrointestinal tract perforation (11). Despite recurrent infections, immunoglobulin levels in these children were generally within normal range. Lymphocyte subpopulation analysis suggested a possible NK cell decline (11/18), memory B-cell decline (7/18), or normal status (7/18).
A total of 16 patients underwent endoscopic assessment, including one patient without gastrointestinal symptoms. Gastroscopic abnormalities included antral ulcers (2/12), gastric lesser curvature ulcers (1/12), esophageal ulcers (1/12), and pharyngeal ulcers (1/12). Colonoscopic abnormalities included total colonic ulcers (6/16), terminal ileal ulcers (6/16), ileocecal valve/ileocecal region ulcers (5/16), transverse colon ulcers (1/16), descending colon ulcers (1/16), and ascending colonic strictures (2/16). Therefore, gastrointestinal ulcers are relatively common in DEX. Even in the absence of clear gastrointestinal symptoms, an initial evaluation using gastroscopy and colonoscopy may be informative for determining the extent and location of ulceration in the gastrointestinal tract and supporting therapeutic decisions (14).
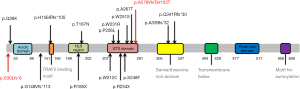
Whole-exome sequencing was performed for all patients and variations in the ELF4 gene were detected (Figure 5). In the majority of cases (22/24), these mutations were inherited from their mothers. To date, 18 variant sites in the ELF4 gene have been discovered, including missense mutations (7/18), frameshift mutations (6/18), nonsense mutations (3/18), deletions (1/18), and intron mutations (1/18). Among these, the most common mutation was the c.700C>T (p.R234X) mutation. Two novel frameshift variants were identified in these patients. Although functional tests have not been conducted yet, convincing evidence suggests that these mutations are pathogenic. To date, neither the Human Gene Mutation Database nor the ClinVar database has presented related reports regarding these variants.
Among the treatment regimens, the majority of patients receive combination drug therapy, whereas a minority undergo single-drug treatment. The outcomes of most patients improve significantly (20/22). Glucocorticoids (21/23) and biologics (16/23) are most commonly used in patients receiving DEX (6). These monoclonal antibodies include those targeting IL-1β (canakinumab), IL-12/23 p40 (ustekinumab), TNF-α (adalimumab and infliximab), and B-cell-activating factor (belimumab). Most reported cases have utilized biologics targeting TNF-α, such as infliximab or adalimumab, and TNF-α has demonstrated favorable outcomes (2,6,7,11-14). In the management of recurrent infections, patients with p.W231R or p.R185X mutations have shown a significant reduction in infections with periodic intravenous immunoglobulin (IVIG) (2,6,8). In the case of patient 1, although respiratory tract infections were noted, they were usually mild; therefore, IVIG therapy was not initiated.
In clinical practice, DEX is often misdiagnosed as Behçet’s disease or IBD due to its overlapping features of autoimmune and AIDs; therefore, careful differential diagnosis is essential. Compared with Behçet’s disease, children with DEX are less likely to present with related manifestations such as ocular vasculitis and central nervous system, cardiac, renal, and vulvar involvement (14). In contrast to IBD, although patients with DEX are prone to complications such as perianal ulcers and abscesses, they are more likely to have symptoms such as skin involvement and recurrent oral ulcers. Moreover, the endoscopic pathological manifestation of DEX is vasculitis, rather than non-caseous granulomas or crypt abscesses.
Conclusions
Given the scarcity of reports on DEX, current understanding of DEX remains limited. In this study, we present detailed clinical data of two children with newly discovered ELF4 variations, thereby contributing to the expanding phenotypic and genotypic spectrum of DEX. Clinicians should consider DEX in male children presenting with recurrent fever, oral ulcers, high levels of inflammatory markers, and digestive tract symptoms. Further endoscopic examinations and genetic tests are required to confirm the presence of this disease.
Acknowledgments
We thank the patients and their families for their participation in the study.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-167/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-167/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-167/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The patients and their family provided informed consent and subsequently underwent whole-exome sequencing. Publication of this case report and accompanying images was waived from patient consent according to the Medical Ethics Committee of Children’s Hospital, Zhejiang University School of Medicine (No. 2014-IRB-0386-P-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tangye SG, Al-Herz W, Bousfiha A, et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol 2022;42:1473-507. [Crossref] [PubMed]
- Sun G, Wu M, Lv Q, et al. A Multicenter Cohort Study of Immune Dysregulation Disorders Caused by ELF4 Variants in China. J Clin Immunol 2023;43:933-9. [Crossref] [PubMed]
- Suico MA, Shuto T, Kai H. Roles and regulations of the ETS transcription factor ELF4/MEF. J Mol Cell Biol 2017;9:168-77. [Crossref] [PubMed]
- Yamada T, Park CS, Mamonkin M, et al. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Krüppel-like factors KLF4 and KLF2. Nat Immunol 2009;10:618-26. [Crossref] [PubMed]
- Lacorazza HD, Miyazaki Y, Di Cristofano A, et al. The ETS protein MEF plays a critical role in perforin gene expression and the development of natural killer and NK-T cells. Immunity 2002;17:437-49. [Crossref] [PubMed]
- Du HQ, Zhao XD. Current understanding of ELF4 deficiency: a novel inborn error of immunity. World J Pediatr 2024;20:444-50. [Crossref] [PubMed]
- Tyler PM, Bucklin ML, Zhao M, et al. Human autoinflammatory disease reveals ELF4 as a transcriptional regulator of inflammation. Nat Immunol 2021;22:1118-26. [Crossref] [PubMed]
- Sun G, Qiu L, Yu L, et al. Loss of Function Mutation in ELF4 Causes Autoinflammatory and Immunodeficiency Disease in Human. J Clin Immunol 2022;42:798-810. [Crossref] [PubMed]
- Salinas SA, Mace EM, Conte MI, et al. An ELF4 hypomorphic variant results in NK cell deficiency. JCI Insight 2022;7:e155481. [Crossref] [PubMed]
- Wang N, Xie Y, Wang Z. Two cases of Behcet’s Disease-Like syndrome with gene deficiency in ELF4. Journal of Sichuan University 2024;55:756-61. [Crossref] [PubMed]
- Zhou Y, Wang L, Zhang C, et al. Two cases of deficiency in ELF4 gene X-linked and literature review. Zhonghua Er Ke Za Zhi 2024;62:1164-8. [Crossref] [PubMed]
- Lv Q, Li Y, Wei Q, et al. Autoinflammatory syndromes mimicking Behçet's disease with gastrointestinal involvement: a retrospective analysis. Clin Exp Rheumatol 2024;42:2076-85. [Crossref] [PubMed]
- Sun L, Han Y, Li B, et al. A Novel Frameshift Variant of the ELF4 Gene in a Patient with Autoinflammatory Disease: Clinical Features, Transcriptomic Profiling and Functional Studies. J Clin Immunol 2024;44:127. [Crossref] [PubMed]
- Olyha SJ, O'Connor SK, Kribis M, et al. "Deficiency in ELF4, X-Linked": a Monogenic Disease Entity Resembling Behçet's Syndrome and Inflammatory Bowel Disease. J Clin Immunol 2024;44:44. [Crossref] [PubMed]


