
Clinical and variant spectrum of patients harboring ATAD3A variants
Highlight box
Key findings
• The discovery of patients harboring new ATAD3A variants expands the variant spectrum and clinical landscape. The clinical course of patients harboring ATAD3A variants is related to the variant type. Prenatal genetic consultation is necessary in families with ATAD3A variants.
What is known and what is new?
• ATAD3A deficiency may lead to respiratory chain deficits. Patients with different ATAD3A variants may exhibit distinct clinical features.
• The article reports additional cases of patients harboring the ATAD3A variant, including new phenotypes and variants. The clinical course of patients harboring ATAD3A variants depends highly on the specific variant type.
What is the implication, and what should change now?
• The findings in this study could inform the genetic consultation for families with ATAD3A variants.
Introduction
The human ATAD3A gene, together with its paralogues ATAD3B and ATAD3C, belongs to the ATPase family AAA domain-containing 3A gene cluster. ATAD3A is involved in a diversity of cellular processes, including but not limited to mitochondrial structure regulation, dynamics control, and cholesterol metabolism regulation (1,2). Variants affecting the ATAD3A gene result in Harel-Yoon syndrome (MIM #617183), which can be inherited in either a dominant manner or recessive manner (3); meanwhile, pontocerebellar hypoplasia, hypotonia, and respiratory insufficiency syndrome, neonatal lethal (MIM #618810), which is inherited in a recessive manner.
As one of the genes encoding proteins involved in the mitochondrial respiratory chain, ATAD3A dysfunction may lead to respiratory chain deficits, resulting in the accumulation of metabolic intermediates or disruption of the integrity of the mitochondrial network (4-6). Thus, heterogeneous clinical features are often observed in patients with ATAD3A variants (7-9). To date, more than 80 patients with ATAD3A variants have been reported since 2016—when ATAD3A was first described as a disease-causing gene (3)—with the majority having a poor prognosis. Therefore, it is important to summarize the reported cases to identify potential genotype–phenotype correlations, which may aid in earlier prognostic evaluation in patients harboring ATAD3A variants.
In this paper, we report three families with ATAD3A variants and analyzed the clinical characteristics and variants of patients with ATAD3A variants reported thus far. The overall aim of the study was to further characterize the clinical and variant landscape of these patients and determine the correlations between genotype and phenotype. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-60/rc).
Methods
Patient and data collection
This observational study examined patients harboring ATAD3A variants who attended Children’s Hospital of Zhejiang University School of Medicine, as well as similar patients from other centers reported in the literature. Between January 2016 and May 2024, five patients harboring ATAD3A variants were recruited from the Children’s Hospital of Zhejiang University School of Medicine, and their clinical data were extracted from the medical records. This study was approved by the Ethics Committee of the Children’s Hospital of Zhejiang University School of Medicine (No. 2024-IRB-0291-P-01). Informed consent was obtained from the guardians of all five probands. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The data of other patients harboring ATAD3A variants were collected by searching four electronic databases [Wangfang, China National Knowledge Infrastructure (CNKI), Chongqing VIP (CQVIP), and PubMed] with the keyword “ATAD3A”; the search period was from database inception to April 30, 2024. Patients without available information were excluded. Data extraction from included reports was performed independently by two reviewers (H.M. and X.Z.), with discrepancies resolved through discussion. A comprehensive analysis of the clinical features and variant spectrum of patients harboring ATAD3A variants was conducted.
Clinical information of case patients
Over the inclusion period, three families with members harboring ATAD3A variants who attended Children’s Hospital of Zhejiang University School of Medicine were identified.
Family 1
There were two affected sisters from family 1. Patient 1-1 was born full-term from the first pregnancy, with normal Apgar scores. She was sent to our hospital due to poor responsiveness at 18 days old. She was in a coma, and hypotonia was noted. Blood gas analysis revealed a pH less than 7.0 and an elevated lactate level (24.0–29.0 mmo1/L). Moreover, lactate dehydrogenase, creatine kinase, and creatine kinase isoenzyme activity were elevated to 1,954, 1,026, and 539 U/L, respectively. Chest X-ray showed exudative changes in the both lungs and a large heart shadow. The clinical course progressed rapidly, and she died from neonatal sepsis and cardiac insufficiency on the first day of admission. Patient 1-2 was the younger sister of patient 1-1. She was full-term (39+2 weeks’ gestation), with a birth weight of 3,170 g and normal Apgar scores. She was admitted to our hospital due tachypnea at 11 days old. Laboratory tests revealed elevated levels N-terminal pro-brain natriuretic peptide (28,037.7 pg/mL; normal range: <300.0 pg/mL), myoglobin (101.5 ng/mL; normal range: 2.75–31.03 ng/mL), creatine kinase isoenzyme (8.9 ng/mL; normal range: <5.0 ng/mL), and plasma lactate (4.0 mmol/L; normal range: 0.5–1.6 mmol/L), along with a pH <7.0. Echocardiography revealed hypertrophic cardiomyopathy and an ejection fraction of 32–69%. Cardiac magnetic resonance showed a thickened left ventricular myocardium with partial fibrosis. Electrocardiography included T-wave and ST changes in the high lateral wall. Chest X-ray revealed increased density in the right lower lobe. Cranial magnetic resonance showed hyperintense T2 signals in the white matter and pons, along with widened extracerebral space in the frontal and temporal regions. Electroencephalography was normal. During hospitalization, recurrent heart failure, hyperlactatemia, feeding difficulties, and failure to thrive were noted. The patient died of cardiopulmonary failure at 3.5 months of age.
Family 2
A boy in family 2 was born at 38 weeks’ gestation from the second pregnancy, with a birth weight of 3,100 g. His Apgar score was 8/5/9, and he had moderate birth asphyxia. Soon after birth, recurrent asphyxia, feeding difficulties, and intractable epilepsy developed. He was transferred to our hospital at 1 month of age and required continuous mechanical ventilation, nasogastric feeding, and antiepileptic therapy. Electroencephalography showed epileptic seizures with a burst-suppression pattern. Cranial magnetic resonance revealed brain atrophy, bilateral symmetrical abnormal signals in the basal ganglia and thalamus, and dilated supratentorial ventricles. Plasma lactate levels ranged from 0.8 to 3.9 mmol/L (normal range: 0.5–1.6 mmol/L). Hearing testing indicated profound hearing loss bilaterally (>99 dBnHL; normal range: <30 dBnHL). Metabolic tests, echocardiography, and the ophthalmologic examinations were unremarkable. The boy died of respiratory failure at 4 months of age.
Family 3
There were two affected siblings from family 3. Patient 3-2, the first child, was born preterm (30+5 weeks’ gestation) due to fetal distress during the second pregnancy, with a birth weight of 1,350 g and Apgar scores of 8/9. Respiratory distress and purulent meningitis developed shortly after birth, followed by poor responsiveness and neonatal seizures. Metabolic tests revealed hyperlactacidemia (20 mmol/L; normal range: 0.5–1.6 mmol/L), hyperammonemia (200 µmol/L; normal range: 9–30 mol/L), metabolic acidosis, elevated amino acids (citrulline, phenylalanine, methionine, tyrosine, alanine, and proline), and mildly increased levels of malonic acid and 4-hydroxybutyric acid. Electroencephalography showed a burst-suppression pattern with epileptic seizures. Cranial magnetic resonance findings included cerebellar hypoplasia and intracranial hemorrhage. Echocardiography revealed noncompaction of the ventricular myocardium with severely reduced left ventricular systolic function. Additional findings included cloudy corneas and hyperparathyroidism. Despite administration of mechanical ventilation, antibiotics, L-carnitine, coenzyme Q10, and supportive care, the patient died of cardiopulmonary failure at 45 days of age.
Patient 3-3, the younger brother, was born preterm (33+2 week’ gestation) due to fetal distress during the third pregnancy, with a birth weight of 2,140 g and Apgar scores of 8/8. Respiratory distress and poor responsiveness were noted at birth. Other findings included cloudy corneas, hearing loss, hemophilia A, neonatal hypoglycemia, and metabolic acidosis. Plasma lactate levels ranged from 0.5 to 8.1 mmol/L (normal range: 0.5–1.6 mmol/L). Echocardiography indicated atrial septal defect and patent ductus arteriosus, while electroencephalography revealed a burst-suppression pattern without seizures. Cranial magnetic resonance indicated brain injury signals and scalp hematoma. Mechanical ventilation, factor VIII replacement, and supportive therapies were administered. The patient died at 19 days of age following withdrawal of treatment.
Whole-exome sequencing and evaluation of variants in case patients
DNA was extracted from whole-blood samples of patients from the three family via the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Genomic DNA fragments were enriched (Roche Nimble Gen Seq EZ Exome Enrichment Kit, Roche, Basel, Switzerland) for whole-exome library preparation. Whole-exome sequencing was performed on an NovaSeq 6000 platform (Illumina, San Diego, CA, USA), achieving ≥99% target coverage at 150× depth. Cleaned reads were aligned to the UCSC hg19 reference genome. Variant calling and analysis were conducted according to established protocols (10,11). Sanger sequencing was used to validate candidate variants. Primers were designed with Primer 6 software, and polymerase chain reaction was conducted with the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). Polymerase chain reaction products were sequenced on an ABI 3130XL genetic analyzer (Applied Biosystems, Foster City, CA, USA) and analyzed with DNA Sequence Analysis Software (http://www.genecodes.com). Finally, copy number variants (CNVs) were identified through WES data analysis, and an evaluation of variant pathogenicity was completed according to the American College of Medical Genetics and Genomics (ACMG) guidelines (12-14).
Statistical analyses
Statistical analyses were performed with SPSS 20.0 (IBM Corp., Armonk, NY, USA). Categorical variables are summarized as frequencies and proportions. The chi-squared test and Fisher exact test were applied for comparisons, with P<0.05 indicating a statistically significant difference.
Results
Phenotypes and genotypes of the patients
We encountered five patients from three families (Figure 1A). All developed symptoms in the fetal or neonatal period and died at between the ages of 18 days and 4 months. The two patients in family 1 were term-born with uneventful pregnancies, the patient from family 2 was term-born with birth asphyxia, and the two patients from family 3 were born preterm due to fetal distress. In family 1 (these two patients were also reported in Journal of Zhejiang University, Medical Sciences in 2023) (15), patient 1-1 presented with cardiac insufficiency and hyperlactatemia and died at 18 days of age from neonatal sepsis and cardiac insufficiency. Patient 1-2 exhibited similar symptoms of hypertrophic cardiomyopathy, hyperlactatemia, and feeding difficulties and died at 3.5 months of age due to cardiopulmonary failure. In family 2, patient 2-2 presented with feeding difficulties, intractable epilepsy, recurrent asphyxia, and brain atrophy and died at 4 months of age from respiratory failure. In family 3, patient 3-2 presented with poor response, respiratory distress, cerebellar hypoplasia, neonatal seizure, hyperlactatemia, cloudy corneas, and noncompaction of the ventricular myocardium and died at 45 days of age due to cardiopulmonary failure. Patient 3-3 presented with poor responsiveness, respiratory distress, hemophilia A, neonatal hypoglycemia, and cloudy corneas and died at 19 days of age following treatment withdrawal. The rapid progression and multiorgan dysfunction in these patients suggested underlying genetic disorders.
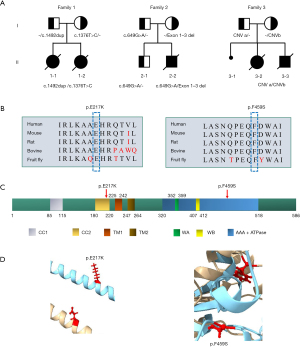
Biallelic variants related with ATAD3A were identified in all five patients (Figure 1A). In family 1, compound heterozygous variants were detected, which were inherited from the father [NM_001170535.3:c.1492dup (p.T498Nfs*13)] and mother [NM_001170535.3:c.1376T>C (p.F459S)], both in exon 14 of ATAD3A. In family 2 patient 2-2 carried a paternally inherited missense variant (NM_001170535.3:c.649G>A, p.E217K) in exon 6 and a maternally inherited deletion (1p36.33:1447542-1452792) spanning exons 1–3 of ATAD3A; his brother (2-1) carried only the c.649G>A variant. In family 3, both patients harbored a paternally inherited 73.7-kb CNV [chr1:1386018-1414136 (duplication); 1417507-1422059 [deletion]; 1447638-1452803 (deletion); 1455510-1459788 (duplication)] and a maternally-inherited 35.3-kb CNV [chr1:1417507-1452803 (deletion)] in 1p36.33. None of variants encountered in these families have been reported previously.
According to the American College of Medical Genetics and Genomics (ACMG) guidelines, the c.1492dup variant is classified as pathogenic, as it is a frameshift variant in ATAD3A whose loss of function is a known mechanism of disease (PVS.1). It is absent from controls (PM2) and is in cosegregation with disease in multiple affected family members (PP1). The exon 1–3 deletion is classified as likely pathogenic, as this deletion involves the 5’ region, and deletions involving the 5′ region of in ATAD3A are associated with specific phenotypes. Both the 73.7-kb CNV and the 35.3-kb CNV in 1p36.33 are classified as pathogenic, as the 73.7-kb CNV results in duplication of the whole ATAD3C gene, partial duplication/deletion of ATAD3B (exons 1–3 duplicated, exons 6–11 deleted), and partial deletion/duplication of ATAD3A (exons 1–3 deleted, exons 6–11 duplicated); meanwhile, the 35.3-kb CNV results in deletion of exons 6–16 in ATAD3B and deletion of exons 1–3 in ATAD3A. The missense variants c.1376T>C and c.649G>A (Figure 1B-1D) are classified as likely pathogenic, as both alter highly conserved residues within critical functional domains (p.E217K may disrupt the ATP-binding pocket; p.F459S may destabilize the hydrophobic core adjacent to transmembrane helices) (PM1). They are absent from controls (PM2), are detected in trans with pathogenic/likely pathogenic variants (PM3), and are predicted by computational prediction tools (PP3) to contribute to a deleterious effect on the gene.
Demographic and clinical characteristics of the patients
A total of 88 patients harboring ATAD3A variants were included, consisting of the five newly reported patients and 83 patients identified from the literature (Table 1). Among them, 55.7% were male and 76.6% were term-born. According to the data available, birth asphyxia was recorded in 55.0% (33/60) of cases, and 51.4% (38/74) had a positive family history. In terms of the age at onset, most patients developed symptoms prenatally (26/71, 36.6%) or neonatally (28/71, 39.4%). Dysmorphic facies were observed in 57.4% (27/47) of patient, while hyperlactatemia, hypertrophic cardiomyopathy, feeding difficulties and seizures were reported in 74.2% (49/66), 54.5% (36/66), 61.5% (24/39), and 61.7% (37/60) of patients, respectively. Hypotonia was present in 94.0% (47/50) of patients, while ocular abnormalities occurred in 93.0% (66/71), with the most frequent being cloudy corneas (30/66, 45.5%) and cataracts (29/66, 43.9%), followed by strabismus (11/66, 16.7%) and nystagmus (7/66, 10.6%). Abnormal brain development was observed in 53.9% (41/76) of patients, predominantly cerebellar hypoplasia (22/41, 53.7%), followed by pontocerebellar hypoplasia (10/41, 24.4%), brain atrophy (7/41, 17.1%), and delayed myelination (5/41, 12.2%). At the last follow-up, only 31.8% (28/88) of patients were alive, and among the survivors, 88.9% (24/27; developmental data unavailable for 1 patient) exhibited developmental delay. Among the 68.2% (60/88) of patients who died, 45% (27/60) succumbed during the perinatal period.
Table 1
| Characteristics† | Value (n=88) |
|---|---|
| Male | 49 (55.7) |
| Term-born (n=64) | 49 (76.6) |
| Birth weight (g) (n=40) | 2,752±572 |
| Caesarean section delivery (n=36) | 26 (72.2) |
| Birth asphyxia (n=60) | 33 (55.0) |
| Positive family history (n=74) | 38 (51.4) |
| Age at onset (n=71) | |
| Fetal period | 26 (36.6) |
| Neonatal period | 28 (39.4) |
| Infant period | 13 (18.3) |
| After infancy | 4 (5.6) |
| Dysmorphic faces (n=47) | 27 (57.4) |
| Ocular abnormalities‡ (n=71) | 66 (93.0) |
| Cloudy corneas | 30 (45.5) |
| Cataracts | 29 (43.9) |
| Strabismus | 11 (16.7) |
| Nystagmus | 7 (10.6) |
| Myopia | 6 (9.1) |
| Optic nerve atrophy | 4 (6.1) |
| Ptosis | 4 (6.1) |
| Hyperlactatemia (n=66) | 49 (74.2) |
| Hypertrophic cardiomyopathy (n=66) | 36 (54.5) |
| Feeding difficulties (n=39) | 24 (61.5) |
| Seizures (n=60) | 37 (61.7) |
| Muscle tone (n=50) | |
| Hypotonia | 47 (94.0) |
| Hypertonia | 3 (6.0) |
| Abnormal brain development§ (n=76) | 41 (53.9) |
| Cerebellar hypoplasia | 22 (53.7) |
| Pontocerebellar hypoplasia | 10 (24.4) |
| Brain atrophy | 7 (17.1) |
| Delayed myelination | 5 (12.2) |
| Brainstem hypoplasia | 3 (7.3) |
| Spinal cord hypoplasia | 3 (7.3) |
| Outcomes | |
| Alive | 28 (31.8) |
| Death | 60 (68.2) |
| Deceased during perinatal period | 27 (45.0) |
| Deceased during newborn period¶ | 11 (18.3) |
| Deceased during infant period | 15 (25.0) |
| Deceased after infancy | 7 (11.7) |
Data are presented as n (%) or mean ± standard deviation. †, characteristics analyzed among patients with available clinical data. ‡, some patients presented multiple symptoms. §, abnormalities detected via brain imaging/autopsy; some patients had multiple findings. ¶, newborn period here refers >7 days but ≤28 days old.
Variant spectrum of the patients
A total of 54 variants were identified in 88 patients (Table S1), including 16 missense variants, 6 frameshift variants, 1 nonsense variant, 1 splice donor variant, 19 deletions, 10 duplications, and 1 rearrangement. Additionally, an abnormal karyotype (47, XXY) was detected. The top three most frequent variants were NC_000001.10:g.1391996_1460043 duplication (detected in 12 patients), NM_001170535.1:c.1582C>T (p.R528W; detected in 8 patients), and NM_001170535.3:c.229C>G (p.L77V; detected in 6 patients). Twenty-nine variants were detected in more than one patient.
Among the 88 patients, 38 (38/88, 43.2%) carried monoallelic variants: 14 with missense variants (one patient had 47, XXY karyotype associated with Klinefelter syndrome, which was excluded from analysis due to its typically asymptomatic presentation before puberty), 1 with nonsense variant, and 23 with duplications. The remaining 50 (50/88, 56.8%) patients had biallelic variants, including 31 patients with compound heterozygous variants (predominantly: missense + frameshift variants in 9 patients, missense + deletion in 8 patients, and deletion + deletion in 4 patients) and 19 patients with homozygous variants (9 with missense variants, 9 with deletions, and 1 with a splice donor variant).
Clinical characteristics of variant subgroups
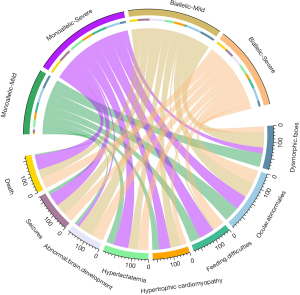
To investigate the clinical phenotypic differences between patients carrying distinct variant types (Table S1), we classified 64 cases (64/88, 72.7%) into a severe group (patients harboring frameshift variants, nonsense variants, splice donor variants, or large deletions/duplications). The remaining 24 patients (24/88, 27.3%) were categorized into the mild group, comprising 22 cases with missense variants only and 2 cases with small deletions (1 causing loss of a single amino acid and the other leading to the loss of 7 amino acids). In the severe group, 17 (17/23, 73.9%) monoallelic patients and 8 (8/21, 38.1%) biallelic patients presented with hypertrophic cardiomyopathy (73.9% vs. 38.1%; P=0.02). Additionally, 4 (4/22, 18.2%) monoallelic patients and 27 (27/35, 77.1%) biallelic patients presented with abnormal brain development (18.2% vs. 77.1%; P<0.001). In the mild group, two (2/11, 18.2%) monoallelic patients and eight (8/8, 100%) biallelic patients presented with abnormal brain development (18.2% vs. 100%; P=0.001). By the last follow-up, one (1/13, 7.7%,) monoallelic patient and six (6/11, 54.5%) biallelic patients died (7.7% vs. 54.5%, P=0.02). Neither the mild group nor the severe group showed significant differences between monoallelic and biallelic patients in terms of dysmorphic faces (P>0.99 and P>0.99), ocular abnormalities (P>0.99 and P=0.70), feeding difficulties (P=0.057 and P=0.27), hyperlactatemia (P>0.99 and P=0.07), or seizures (P=0.057 and P=0.22) (Table 2). In addition, 50 patients had biallelic variants and 38 patients had monoallelic variants. Among the monoallelic patients, nine (9/10, 90.0%) with mild variants and five (38.5%, 5/13) with severe variants presented with dysmorphic faces (90.0% vs. 38.5%; P=0.03); four (4/8, 50.0%) with mild variants and 19 (19/20, 95.0%) with severe variants presented with hyperlactatemia (50.0% vs. 95.0%; P=0.02); two (2/9, 22.2%) with mild variants and 11 (11/13, 84.6%) with severe variants presented with seizures (22.2% vs. 84.6%; P=0.007); by the last follow-up, 1 (7.7%, 1/13) with a mild variant and 24 (96.0%, 24/25) with severe variants had died (7.7% vs. 96.0%; P<0.001). Among biallelic patients, six (6/6, 100.0%) with mild variants and seven (7/18, 38.9%) with severe variants presented with dysmorphic faces (100.0% vs. 38.9%; P=0.02). No significant differences were observed in either the monoallelic or biallelic groups between mild and severe variants in terms of ocular abnormalities (P>0.99 and P=0.56), feeding difficulties (P=0.26 and P=0.06), hypertrophic cardiomyopathy (P=0.21 and P=0.14), or abnormal brain development (P>0.99 and P=0.32) (Table 3). We drew a circos plot to provide an enhanced visual interpretation of the different phenotype found across the groups (Figure 2).
Table 2
| Characteristics | Mild | Severe | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Monoallelic | Biallelic | χ2 | P | Monoallelic | Biallelic | χ2 | P | ||
| Dysmorphic faces | 9/10 (90.0) | 6/6 (100.0) | – | >0.99 | 5/13 (38.5) | 7/18 (38.9) | – | >0.99 | |
| Ocular abnormalies | 10/10 (100.0) | 11/11 (100.0) | – | >0.99 | 18/19 (94.7) | 27/31 (87.1) | 0.151 | 0.70 | |
| Feeding difficulties | 8/12 (66.7) | 1/7 (14.3) | – | 0.057 | 5/5 (100.0) | 10/15 (66.7) | – | 0.27 | |
| Hypertrophic cardiomyopathy | 4/9 (44.4) | 7/10 (70.0) | – | 0.37 | 17/23 (73.9) | 8/21 (38.1) | 5.74 | 0.02 | |
| Hyperlactatemia | 4/8 (50.0) | 5/8 (62.5) | – | >0.99 | 19/20 (95.0) | 21/30 (70.0) | 3.255 | 0.07 | |
| Abnormal brain development | 2/11 (18.2) | 8/8 (100.0) | – | 0.001 | 4/22 (18.2) | 27/35 (77.1) | 16.63 | <0.001 | |
| Seizures | 2/9 (22.2) | 6/8 (75.0) | – | 0.057 | 11/13 (84.6) | 18/30 (60.0) | 1.507 | 0.22 | |
| Death | 1/13 (7.7) | 6/11 (54.5) | – | 0.02 | 24/25 (96.0) | 29/39 (74.4) | 3.608 | 0.058 | |
Data are presented as n/N (%). Severe group: patients harboring frameshift variants, nonsense variants, splice donor variants, or large deletions/duplications; Mild group: comprising 22 cases with missense variants only and 2 cases with small deletions (one causing loss of a single amino acid and the other leading to loss of seven amino acids).
Table 3
| Characteristics | Monoallelic | Biallelic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Severe | χ2 | P | Mild | Severe | χ2 | P | ||
| Dysmorphic faces | 9/10 (90.0) | 5/13 (38.5) | – | 0.03 | 6/6 (100.0) | 7/18 (38.9) | – | 0.02 | |
| Ocular abnormalies | 10/10 (100.0) | 18/19 (94.7) | – | >0.99 | 11/11 (100.0) | 27/31 (87.1) | – | 0.56 | |
| Feeding difficulties | 8/12 (66.7) | 5/5 (100.0) | – | 0.26 | 1/7 (14.3) | 10/15 (66.7) | – | 0.06 | |
| Hypertrophic cardiomyopathy | 4/9 (44.4) | 17/23 (73.9) | – | 0.21 | 7/10 (70.0) | 8/21 (38.1) | – | 0.14 | |
| Hyperlactatemia | 4/8 (50.0) | 19/20 (95.0) | – | 0.02 | 5/8 (62.5) | 21/30 (70.0) | – | 0.69 | |
| Abnormal brain development | 2/11 (18.2) | 4/22 (18.2) | – | >0.99 | 8/8 (100.0) | 27/35 (77.1) | – | 0.32 | |
| Seizures | 2/9 (22.2) | 11/13 (84.6) | – | 0.007 | 6/8 (75.0) | 18/30 (60.0) | – | 0.68 | |
| Death | 1/13 (7.7) | 24/25 (96.0) | – | <0.001 | 6/11 (54.5) | 29/39 (74.4) | 1.604 | 0.21 | |
Data are presented as n/N (%). Severe group: patients harboring frameshift variants, nonsense variants, splice donor variants, or large deletions/duplications; Mild group: comprising 22 cases with missense variants only and 2 cases with small deletions (one causing loss of a single amino acid and the other leading to loss of seven amino acids).
Discussion
Since Harel-Yoon syndrome was first described in 2016 (3), patients harboring ATAD3A variants have garnered heightened research attention. Thus far, 88 patients harboring ATAD3A variants have been identified, including the 5 cases reported here. The clinical features of these patients are highly heterogeneous. Our patients exhibited common characteristics previously reported, such as hypertrophic cardiomyopathy, hyperlactatemia, feeding difficulties, seizures, and cloudy corneas (7-9,16,17). Additionally, we observed novel phenotypes, such as noncompaction of ventricular myocardium and recurrent asphyxia, expanding upon the phenotype spectrum of patients harboring ATAD3A variants. Consistent with prior reports of severe outcomes (8,18,19), all our patients experienced rapid progression and died between 18 days to 4 months.
To characterize the clinical heterogeneity and the expanding phenotypic spectrum, we systematically reviewed reported patients harboring ATAD3A variants. Although the onset time was previously described as being primarily in infancy (3,9,20), our analysis of exact onset ages revealed that approximately three-quarters of patients presented prenatally or neonatally, with only 5.6% doing so after infancy. Ocular abnormalities, observed in 93.0% of patients (3,7,8,16,21,22), were predominantly cloudy corneas (45.5%) and cataracts (43.9%), underscoring the importance of ophthalmologic evaluation. Brain imaging and electroencephalography are critical ancillary tests, and abnormal brain development was observed in more than half of the patients (with cerebellar being the most vulnerable part) (7,19,21,23), and seizures occurred in about two-thirds of the patients (7,8,19,21,24). Other prevalent features present in over half of the patients were dysmorphic facies, hyperlactatemia, hypertrophic cardiomyopathy, hypotonia, and feeding difficulties (3,9,16,22). Outcomes were poor (3,8,9,16,18), with fewer than one-third of the patients surviving to the last follow-up (most survivors had developmental delay). Among the deceased patients, about 90% died within the first year of life, nearly half during the perinatal period. Thus, ATAD3A should be considered a critical gene in prenatal screening, with prenatal genetic consultation being essential for families with ATAD3A variants.
Thus, 54 ATAD3A variants have been reported. Deletions are the most common variant type, followed by missense variants and duplications. The 3 most common variants and the 29 variants detected in more than one patient warrant attention in further genetic testing interpretation. Since more than half of the patients were with biallelic variants, recessive mode appears to be more common. Moreover, we reported six novel variants, including two missense variants (c.1376T>C and c.649G>A), one frameshift variant (c.1492dup), and three CNVs. Based on the ACMG guidelines (12-14), all of them were classified as pathogenic/likely pathogenic, which should be noted in the future identification of such patients.
The variable clinical spectrum implies the presence of genotype–phenotype correlations (17,23-26). Thus, we compared the occurrence rates of several phenotypes between patients with different kind of variants. It was found that in the monoallelic group, hyperlactatemia, seizures, and death were more common in severe variants compared to mild variants. This may be due to the fact that severe variants are more likely to cause malfunction of the ATAD3A gene with subsequent severe mitochondrial dysfunction (8,23). Unexpectedly, dysmorphic faces were more common in mild variants in both the monoallelic and biallelic groups, possibly due to early mortality obscuring facial features in severe cases (8,16). Furthermore, monoallelic patients had lower rates of abnormal brain development than did biallelic patients in both mild and severe groups, suggesting this phenotype is likely to be recessive. Conversely, hypertrophic cardiomyopathy was more frequent in severe monoallelic cases, a finding that warrants further investigation.
Limitations
Our study involved several limitations that should be acknowledged. First, the severe–mild variant classification, while operationally useful, may oversimplify functional impact (e.g., critical-domain missense variants could be severe). Second, the limited sample size and incomplete clinical data in some cases might constrain the statistical power of the results, and observations in some subgroups should be interpreted cautiously. Future studies with larger cohorts are needed to validate and refine these findings.
Conclusions
Our study expanded upon the variant spectrum and clarified clinical landscape of patients harboring ATAD3A variants. The clinical course of these patients is highly variable and correlates with variant type. Due to the severe symptoms and poor prognoses associated with its variants, ATAD3A should be a considered a necessary inclusion in prenatal screening and in the prenatal genetic consultation for at-risk families.
Acknowledgments
We express our deep gratitude to the patients and families for their participation.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-60/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-60/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-60/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-60/coif). All authors report that this study was supported by the Pre-research Fund of Children’s Hospital, Zhejiang University School of Medicine (No. CHZJU2023YY010) and the Joint Fund of Zhejiang Provincial Natural Science Foundation of China (No. LKLY25H090001). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Children’s Hospital of Zhejiang University School of Medicine (No. 2024-IRB-0291-P-01). Informed consent was obtained from the guardians of all five probands. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gaudó P, de Tomás-Mateo E, Garrido-Pérez N, et al. ATAD3C regulates ATAD3A assembly and function in the mitochondrial membrane Free Radic Biol Med 2024;211:114-26. [Crossref] [PubMed]
- Arguello T, Peralta S, Antonicka H, et al. ATAD3A has a scaffolding role regulating mitochondria inner membrane structure and protein assembly. Cell Rep 2021;37:110139. [Crossref] [PubMed]
- Harel T, Yoon WH, Garone C, et al. Recurrent De Novo and Biallelic Variation of ATAD3A, Encoding a Mitochondrial Membrane Protein, Results in Distinct Neurological Syndromes. Am J Hum Genet 2016;99:831-45. [Crossref] [PubMed]
- Chen L, Li Y, Sottas C, et al. Loss of mitochondrial ATPase ATAD3A contributes to nonalcoholic fatty liver disease through accumulation of lipids and damaged mitochondria. J Biol Chem 2022;298:102008. [Crossref] [PubMed]
- Dorison N, Gaignard P, Bayot A, et al. Mitochondrial dysfunction caused by novel ATAD3A mutations. Mol Genet Metab 2020;131:107-13. [Crossref] [PubMed]
- Ezer S, Ronin N, Yanovsky-Dagan S, et al. Transcriptome analysis of atad3-null zebrafish embryos elucidates possible disease mechanisms. Orphanet J Rare Dis 2025;20:181. [Crossref] [PubMed]
- Peralta S, González-Quintana A, Ybarra M, et al. Novel ATAD3A recessive mutation associated to fatal cerebellar hypoplasia with multiorgan involvement and mitochondrial structural abnormalities. Mol Genet Metab 2019;128:452-62. [Crossref] [PubMed]
- Frazier AE, Compton AG, Kishita Y, et al. Fatal perinatal mitochondrial cardiac failure caused by recurrent de novo duplications in the ATAD3 locus. Med 2021;2:49-73. [Crossref] [PubMed]
- Skopkova M, Stufkova H, Rambani V, et al. ATAD3A-related pontocerebellar hypoplasia: new patients and insights into phenotypic variability. Orphanet J Rare Dis 2023;18:92. [Crossref] [PubMed]
- Ye Q, Shen Q, Rao J, et al. Multicenter study of the clinical features and mutation gene spectrum of Chinese children with Dent disease. Clin Genet 2020;97:407-17. [Crossref] [PubMed]
- Zhou F, Mao J, Ye Q, et al. Clinical features and genetic findings in Chinese children with distal renal tubular acidosis. Int J Clin Exp Pathol 2018;11:3523-32.
- Riggs ER, Andersen EF, Cherry AM, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med 2020;22:245-57. [Crossref] [PubMed]
- Abou Tayoun AN, Pesaran T, DiStefano MT, et al. Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat 2018;39:1517-24. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Zheng Y, Yu X, Zhang T, et al. ATAD3A gene variations in a family with Harel-Yoon syndrome. Zhejiang Da Xue Xue Bao Yi Xue Ban 2023;52:738-43. [Crossref] [PubMed]
- Gunning AC, Strucinska K, Muñoz Oreja M, et al. Recurrent De Novo NAHR Reciprocal Duplications in the ATAD3 Gene Cluster Cause a Neurogenetic Trait with Perturbed Cholesterol and Mitochondrial Metabolism. Am J Hum Genet 2020;106:272-9. [Crossref] [PubMed]
- Chen Y, Rong S, Luo H, et al. Ketogenic Diet Attenuates Refractory Epilepsy of Harel-Yoon Syndrome With ATAD3A Variants: A Case Report and Review of Literature. Pediatr Neurol 2023;143:79-83. [Crossref] [PubMed]
- Azova S, Rajabi F, Modi BP, et al. Graves’ disease in a five-month-old boy with an unusual treatment course. J Pediatr Endocrinol Metab 2021;34:401-6. [Crossref] [PubMed]
- Ebihara T, Nagatomo T, Sugiyama Y, et al. Severe spinal cord hypoplasia due to a novel ATAD3A compound heterozygous deletion. Mol Genet Metab Rep 2022;33:100912. [Crossref] [PubMed]
- Lepelley A, Della Mina E, Van Nieuwenhove E, et al. Enhanced cGAS-STING-dependent interferon signaling associated with mutations in ATAD3A. J Exp Med 2021;218:e20201560. [Crossref] [PubMed]
- Yap ZY, Park YH, Wortmann SB, et al. Functional interpretation of ATAD3A variants in neuro-mitochondrial phenotypes. Genome Med 2021;13:55. [Crossref] [PubMed]
- Peeters-Scholte CMPCD, Adama van Scheltema PN, Klumper FJCM, et al. Genotype-phenotype correlation in ATAD3A deletions: not just of scientific relevance. Brain 2017;140:e66. [Crossref] [PubMed]
- Desai R, Frazier AE, Durigon R, et al. ATAD3 gene cluster deletions cause cerebellar dysfunction associated with altered mitochondrial DNA and cholesterol metabolism. Brain 2017;140:1595-610. [Crossref] [PubMed]
- Hanes I, McMillan HJ, Ito Y, et al. A splice variant in ATAD3A expands the clinical and genetic spectrum of Harel-Yoon syndrome. Neurol Genet 2020;6:e452. [Crossref] [PubMed]
- Tawfik CA, Zaitoun R, Farag AA. Harel Yoon syndrome: a novel mutation in ATAD3A gene and expansion of the clinical spectrum. Ophthalmic Genet 2023;44:226-33. [Crossref] [PubMed]
- Al Madhoun A, Alnaser F, Melhem M, et al. Ketogenic diet attenuates cerebellar atrophy progression in a subject with a biallelic variant at the ATAD3A locus. Appl Clin Genet 2019;12:79-86. [Crossref] [PubMed]


