
Epidemiological characteristics and trends in the diagnosis and treatment of fungal otitis externa in children
Highlight box
Key findings
• This study analyzed the epidemiology and treatment trends of pediatric fungal otitis externa over 8 years and found that male predominance, with summer being the peak season for the disease.
What is known and what is new?
• Fungal otitis externa is a common pediatric ear infection and a form of otomycosis that rarely extends to the middle ear.
• The diagnostic approach in the late-visit group shifted from fungal smears to a combination of otoscopy and microbial culture. There was also an increased use of triamcinolone acetonide and econazole nitrate cream due to their broad-spectrum anti-inflammatory properties.
What is the implication, and what should change now?
• We found that seasonal and gender patterns were observed; diagnostic changes influenced treatment; further study of new technologies is needed.
Introduction
Fungal otitis externa, a common pediatric ear infection and a form of otomycosis, rarely extends to the middle ear (1). Its incidence is significantly higher in warm, humid environments, peaking during summer and the swimming season, primarily due to excessive use of antibiotic ear drop (1-3). This condition is often misdiagnosed and persistent, emphasizing the need for accurate diagnosis and standardized treatment protocols. Recent advancements in diagnostic technologies, along with the impact of coronavirus disease 2019 (COVID-19), have influenced its epidemiological characteristics, as well as testing, diagnosis, and treatment strategies. This study aims to analyze the epidemiological characteristics and evolving trends in the diagnosis and treatment of fungal otitis externa in children over a period of 8 years. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-169/rc).
Methods
Participants
Outpatient and emergency department records from the Otorhinolaryngology-Head and Neck Surgery Department were retrieved from the hospital database for pediatric patients diagnosed with fungal otitis externa or external auditory canal fungal infection between January 2016 and December 2023. The dataset included patient demographics (gender, age), visit details (dates, frequency), laboratory test results, diagnoses, and prescribed treatments.
This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the ethics committee of Capital Institute of Pediatrics (No. SHERLLM2025024). The need for informed consent was waived by the ethics committee of Capital Institute of Pediatrics because of the retrospective nature of the study. The requirement of patient consent for inclusion was waived. Patient personal privacy and data confidentiality have been upheld.
Inclusion criteria
Patients who met the following criteria were included in this study: (I) aged 0 to 18 years; (II) presenting with symptoms such as ear itching, ear pain, and otorrhea; (III) physical examination findings of white, curd-like secretions or hyphal growth in the external auditory canal; and (IV) fungal smear of ear secretions, obtained using sterile cotton swabs, demonstrating hyphal structures and/or a positive fungal culture.
Exclusion criteria
Patients were excluded based on the following criteria: (I) repeated specimens submitted by the same patient within a 3-month period, with only the initial specimen retained for analysis; and (II) infections extending to the auricle or middle ear.
Clinical manifestations and diagnosis
The clinical manifestations of fungal otitis externa include pruritus, otalgia, and otorrhea. Among these, deep-seated itching is often the most distressing symptom for the patients. The pain is usually less severe than that seen in bacterial otitis externa. The diagnostic examinations include: (I) otoscopy: a 0° otoscopic examination revealed white, debris-like, cheese-like secretions, or villous hyphal structures in the external auditory canal, indicating fungal otitis externa (Figure 1). (II) Fungal smear: sterile cotton swabs were used to collect secretions from the deep portion of the external auditory canal. The samples were smeared onto slides and sent for microscopic analysis at the dermatology mycology laboratory. A positive result was confirmed upon the visualization of hyphal or spore structures. (III) Fungal culture: secretions were collected using sterile cotton swabs from the deep portion of the external auditory canal, placed in a sterile tube, sealed, and sent for fungal culture and drug sensitivity testing (paper strip method). A positive culture was confirmed after 5 days if fungal growth was detected, which supported the diagnosis of fungal otitis externa. The diagnosis was established based on otoscopic examination findings of canal secretions in combined with fungal smear or culture results.
Statistical analysis
Data analysis was performed using SPSS software (version 27.0). Descriptive statistics were applied for data evaluation. Non-normally distributed measurement data were expressed as the median and interquartile range. Categorical and measurement data were analyzed using nonparametric methods, including the Chi-squared test and the independent-sample Mann-Whitney U test. Statistical significance was defined as P<0.05.
Results
General information
A total of 1,377 children were included in the study, consisting of 751 males (54.5%) and 626 females (45.5%). The number of male patients was significantly higher than female patients (P=0.001) (Table 1). The median age was 2.3 years (range, 0.7–6.2 years) (Table 2).
Table 1
| Groups | Male | Female | χ2 | P value |
|---|---|---|---|---|
| Total (n=1,377) | 751 (54.5) | 626 (45.5) | 11.347† | 0.001** |
| Early-visit group (n=849) | 464 (54.7) | 385 (45.3) | 7.529† | 0.007** |
| Late-visit group (n=528) | 287 (54.4) | 241 (45.6) | 3.963† | 0.045* |
Data are presented as n (%). †, significant difference. *, P<0.05; **, P<0.01.
Table 2
| Metrics | Early-visit group | Late-visit group | χ2/Z | P value |
|---|---|---|---|---|
| Patients | 849 | 528 | χ2=74.830† | <0.001*** |
| Age (years) | 1.5 [0.6, 4.7] | 4.7 [1.3, 8.1] | Z=−10.282 | <0.001*** |
| Frequency of visits (times) | 1 [1, 3] | 1 [1, 2] | Z=−2.806 | 0.005** |
Data are presented as number or median [25% percentile, 75% percentile]. †, significant difference. **, P<0.01; ***, P<0.001.
Monthly patient distribution
Patient data classified by month indicated a significantly higher incidence of fungal otitis externa in July, August, September, and October, with each month recording over 150 cases. Both male and female patients exhibited an increased case count during these months compared to other periods (Figure 2).
Patients were further categorized into quarterly intervals: 250 in the first quarter (January–March), 253 in the second quarter (April–June), 551 in the third quarter (July–September), and 323 in the fourth quarter (October–December). Statistical comparisons revealed a significant increase in patient numbers in the third quarter compared to the first (P<0.001), second (P<0.001), and fourth quarters (P<0.001). Additionally, the fourth quarter showed significantly higher cases than the first (P=0.002) and second quarters (P=0.004).
The peak season for visits occurred from July to October, with the third quarter showing a significantly higher number of cases than the other quarters (P<0.001).
Group comparison
Patient distribution by time group
Patients were divided into two groups based on the visit dates: the early-visit group [2016–2019] and the late-visit group [2020–2023]. The early-visit group consisted of 849 patients, while the late-visit group included 528 patients. Statistical analysis identified a significant difference between the groups (P<0.001), with the early-visit group having a substantially higher patient count (Table 2, Figure 3).
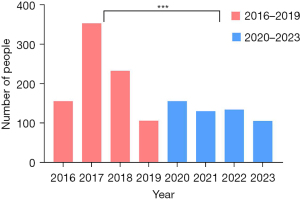
Gender distribution by group
The early-visit group included 464 males (54.7%) and 385 females (45.3%), with a significant gender difference (P=0.007). Similarly, the late-visit group comprised 287 males (54.4%) and 241 females (45.6%), also demonstrating a significant gender disparity (P=0.045). In both groups, males were more frequently diagnosed with fungal otitis externa than females (Table 1).
Number of visits by group
The median number of visits was 1 (range, 1–2). In the early-visit group, the median was 1 visit (range, 1–3), with a maximum of 29 visits. The late-visit group had a median of 1 visit (range, 1–2), with a maximum of 15 visits. A statistically significant difference was noted between the two groups (P=0.005) (Table 2).
Age distribution and differences by groups
The median age of the early-visit group was 1.5 years (range, 0.6–4.7 years), whereas that of the late-visit group was 4.7 years (range, 1.3–8.1 years), with a significant age difference between the two groups (P<0.001). The detailed age distribution is shown in Table 2.
The study population included 435 infants (<1 year), 399 children (1–3 years), 259 preschoolers (4–6 years), 259 school-age children (7–12 years), and 25 adolescents (13–18 years) (Figure 4).
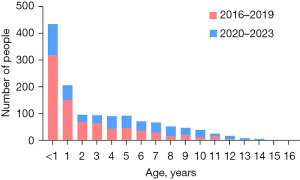
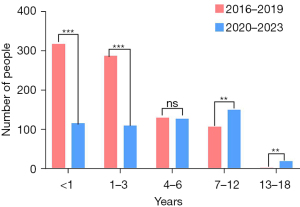
The early-visit group included 318 infants (<1 year) and 288 children (1–3 years), while the late-visit group comprised 117 infants (<1 year) and 111 children (1–3 years), with significant differences between these age categories (P<0.001 for those <1 year; P<0.001 for 1–3 years). In the 4 to 6 years category, the early-visit group had 131 individuals, while the late-visit group had 128, showing no significant difference (P=0.86). Among children aged 7 to 12 years, there were 108 cases in the early-visit group and 151 in the late-visit group, with a statistically significant difference (P=0.008). In the 13 to 18 years category, the early-visit group had four individuals, while the late-visit group had 21, also showing a significant difference (P=0.001). With the exception of the 4 to 6 years category, all other age groups demonstrated significant differences between the early and late-visit groups (Figure 5). The early-visit group predominantly consisted of younger children, particularly infants and toddlers, whereas the late-visit group had a higher proportion of school-age and adolescent children (P<0.001).
Treatment regimen
The treatment regimen included miconazole nitrate cream or a combination of triamcinolone acetonide and econazole nitrate cream. Following ear canal debridement with hydrogen peroxide, the medication was applied using a sterile cotton swab for 7 to 14 days. When fungal smear results were available within a few hours, miconazole nitrate cream was typically prescribed. In the late-visit cohort, where fungal smear was replaced by otoscopy and fungal/bacterial cultures (which required a 5 to 7 days incubation period), patients who presented with characteristic symptoms and visible fungal hyphae under otoscopy were generally treated with miconazole nitrate cream. For patients who exhibited typical symptoms but lacked visible hyphae, triamcinolone acetonide and econazole nitrate creams were commonly used. A wait-and-see approach was adopted for those with atypical symptoms. Miconazole nitrate or triamcinolone acetonide and econazole nitrate cream were administered only after confirmation of a positive fungal culture result.
Discussion
This large-sample study identified a gender-based difference in fungal otitis externa, a topic rarely explored in prior literature, with no statistically significant variation previously report (4-6). Poor hand and fingernail hygiene in males may contribute to increased damage to the ear canal skin barrier. Additionally, a notable rise in fungal otitis externa cases was recorded from July to October, likely attributable to increased temperature and humidity during late summer and early autumn in Beijing, along with a rise in swimming activities during summer vacation, aligning with previous findings (1).
In this study, a larger cohort of children was included in the early-visit group [2016–2019], characterized by a younger age profile and more frequent visits. In contrast, the late-visit group [2020–2023] comprised fewer children, an older age profile, and fewer visits, demonstrating statistically significant differences. Otomycosis in infants and young children is often associated with eczema and skin barrier disruption (5). Among preschool and school-age children, the condition is primarily linked to swimming and prolonged antibiotic ear drop use (2,7).
After multiple nations and the World Health Organization declared the pandemic in March 2020, during the period of COVID-19, preventive measures were implemented to curb virus transmission, including social distancing, school and workplace closures, enhanced hygiene practices, frequent temperature monitoring, and the use of disinfectants, alcohol, and masks. These interventions contributed to a decline in acute otitis media incidence in children (8). The closure of most swimming pools during the outbreak limited swimming opportunities, reducing both antibiotic ear drop use and swimming frequency, which likely contributed to a decrease in otomycosis incidence.
In the late-visit group, the proportion of infants and children aged 0 to 3 years was significantly lower than in the early-visit group. This decline was influenced by limited healthcare access during the outbreak and concerns about hospital-acquired infections, prompting caregivers to opt for home observation. Additionally, the decline in birth rates after 2020 may have further contributed to fewer visits and an older median age in the late-visit group (9). Conversely, the number of school-age children and adolescents increased, possibly due to the rise in online classes and greater headphone use during home isolation.
Moreover, fungal otitis externa has been linked to hearing aid use, ear trauma, tympanic membrane perforation, and a history of mastoid surgery (2). Immunocompromised individuals are particularly vulnerable to this infection, which may also arise following tympanostomy tube placement (8). Although fungal infections can lead to tympanic membrane perforation, most cases resolve with ear debridement and appropriate pharmacological therapy, eliminating the need for surgical intervention (10). Additionally, fungal infections may affect the outcomes of tympanic membrane repair surgeries (11). This study provides a broad analysis of fungal otitis externa using large-scale data, with plans for further investigation of specific cases in future research.
A retrospective study at University Hospital Limerick, Ireland, examined children with positive fungal ear swabs over a 15-year period, identifying Candida as the most prevalent pathogen, followed by Aspergillus (7). This finding is consistent with reports from other institutions, where Candida was the dominant genus (12). Some studies have identified Aspergillus flavus as the primary pathogen, with Candida as the second most common (13,14). Conversely, other research has reported Aspergillus niger as the most frequently encountered genus (1,4,15). In this study, fungal smear testing in the early-visit group detected only spores or hyphae, without identifying the infecting species. In contrast, improved diagnostic technologies in the late-visit group enabled fungal cultures to replace smear testing, providing greater specificity and allowing for targeted antifungal therapy based on pathogen identification. However, the longer culture duration delayed antifungal treatment initiation.
The combination of triamcinolone acetonide and econazole nitrate cream not only possesses antifungal properties but also exhibits broad-spectrum anti-inflammatory effects, providing a broader therapeutic scope than econazole nitrate cream, which primarily targets fungal infections. Antifungal drugs are mainly divided into two classes: polyenes (e.g., amphotericin B and natamycin) and azoles (e.g., imidazoles and triazoles) (16). These medications act by damaging the fungal cell membrane, thereby inhibiting fungal growth and metabolism. Both drug types used in this study contain azoles, which are widely applied in clinical practice (17,18). Additionally, the combination cream containing triamcinolone and econazole not only exerts antifungal effects but also alleviates inflammation; it is considered safe (19), convenient, and well tolerated (20,21). Moreover, drug delivery via irrigation has shown better efficacy compared to topical application alone (22).
Metagenomic next-generation sequencing (mNGS) technology offers distinct advantages in detecting mixed infections and fungal pathogens, serving as a valuable adjunct to conventional microbial culture methods (23). It is increasingly applied in severe infections, including pediatric pneumonia, sepsis, pulmonary fungal infections, and mucormycosis, yet remains underutilized in otomycosis (16-19,24).
Rapid immunofluorescence staining, known for its ease of observation and fast detection, has been employed in lung infections, skin biopsy pathology, and various clinical settings (20-22,25,26). This technique facilitates rapid and accurate diagnosis, with results available within 30 minutes (27). By fluorescently staining fungi and spores, preliminary morphological identification can be performed, making it a complementary or even alternative diagnostic tool to fungal culture (28). However, its application in ear fungal infections remains limited, highlighting the need for further investigation into its potential role in diagnosing fungal otitis externa.
Advancements in diagnostic and therapeutic technologies have progressively increased otoscope utilization in pediatric populations, substantially improving otomycosis diagnosis. Endoscopy, particularly valuable for infants and young children, enhances visualization of fungal hyphae, minimizing diagnostic errors (5). Otoscopic cleaning, when combined with antifungal irrigation, yields superior therapeutic outcomes (29-31). However, commonly available antifungal treatments in paste form restrict effective drug penetration into the deeper ear canal. Future research will investigate the effects of different application methods—smearing versus irrigation—on patient prognosis. A novel drug delivery device, in conjunction with other antifungal ear drop formulations and optimized drug application techniques, presents a more effective strategy for managing fungal otitis externa (32-34). The limitation of this study is that, despite the large sample size, it lacks detailed analysis of specific fungal pathogens, such as Aspergillus and Candida species, including their quantities and characteristics. Additionally, we did not assess how different antifungal drugs and administration methods impact treatment outcomes. These aspects will be the focus of our future research.
Conclusions
This study demonstrated that fungal otitis externa was more prevalent in male children, peaking during the summer, consistent with previous findings. A decline in patient numbers in the late-visit group and an increase in patient age indicated a correlation with reduced swimming opportunities and a decreasing birth rate during the pandemic. Advances in diagnostic methodologies shaped treatment regimens, leading to greater use of triamcinolone acetonide and econazole nitrate cream due to their broad-spectrum anti-inflammatory properties. Future research should investigate the potential of emerging diagnostic technologies, including mNGS and rapid immunofluorescence staining, in enhancing the management of fungal otitis externa.
Acknowledgments
We thank the leaders of the institute’s guidance and the strong support of colleagues in the department during this scientific research and paper collaboration process.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-169/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-169/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-169/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-169/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the ethics committee of Capital Institute of Pediatrics (No. SHERLLM2025024). The need for informed consent was waived by the ethics committee of Capital Institute of Pediatrics because of the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nazari T, Peymaeei F, Ghazi Mirsaid R, et al. Otomycosis: a systematic review and meta-analysis of prevalence and causative agents in the era of molecular diagnostics. BMC Infect Dis 2025;25:544. [Crossref] [PubMed]
- Singh Gill GP, Panchal V, Bakshi R. Fungal otitis externa and tympanic membrane perforation. Indian J Otolaryngol Head Neck Surg 2023;75:1-5. [Crossref] [PubMed]
- Sangaré I, Amona FM, Ouedraogo RW, et al. Otomycosis in Africa: Epidemiology, diagnosis and treatment. J Mycol Med 2021;31:101115. [Crossref] [PubMed]
- Sabz G, Gharaghani M, Mirhendi H, et al. Clinical and microbial epidemiology of otomycosis in the city of Yasuj, southwest Iran, revealing Aspergillus tubingensis as the dominant causative agent. J Med Microbiol 2019;68:585-90. [Crossref] [PubMed]
- Wang DM, Yan YY, Shi YN, et al. Analysis of ear endoscopy for purulent lesions caused by infantile ear discharge. Chinese Journal of Otorhinolaryngology in Integrative Medicine 2022;30:47-8, 5.
- Li GQ, Xu DY, Li CL, et al. Clinical curative effect of endoscopic debridement combined peverson canal perfusion in the treatment of children otomycosis. China Medicine and Pharmacy 2017;7:217-9.
- Westby D, O'Connell N, Powell J, et al. The changing nature of paediatric otomycosis in the mid-west of Ireland. J Laryngol Otol 2020;134:592-6. [Crossref] [PubMed]
- Marom T, Pitaro J, Shah UK, et al. Otitis Media Practice During the COVID-19 Pandemic. Front Cell Infect Microbiol 2021;11:749911. [Crossref] [PubMed]
- Ullah MA, Moin AT, Araf Y, et al. Potential Effects of the COVID-19 Pandemic on Future Birth Rate. Front Public Health 2020;8:578438. [Crossref] [PubMed]
- Peyser-Rosenberg M, Hadar A, Sofer N, et al. Shifting patterns of acute otitis media and mastoiditis through COVID-19 Era: analysis of pre-pandemic, pandemic, and post-pandemic dynamics. Eur Arch Otorhinolaryngol 2025; Epub ahead of print. [Crossref]
- Lou Z. Fungal otitis externa and wet ear with mucopurulent should be influencing factors on tympanic membrane closure. Eur Arch Otorhinolaryngol 2020;277:1557-8. [Crossref] [PubMed]
- Smiianov VA, Ivakhniuk TV, Plakhtiienko IO, et al. The microbiological structure of otomycosis: sensitivity profile of agents to antifungal drugs. Pol Merkur Lekarski 2023;51:42-7. [Crossref] [PubMed]
- Javidnia J, Ghotbi Z, Ghojoghi A, et al. Otomycosis in the South of Iran with a High Prevalence of Tympanic Membrane Perforation: A Hospital-Based Study. Mycopathologia 2022;187:225-33. [Crossref] [PubMed]
- Kiakojuri K, Rajabnia R, Mahdavi Omran S, et al. Role of Clotrimazole in Prevention of Recurrent Otomycosis. Biomed Res Int 2019;2019:5269535. [Crossref] [PubMed]
- Nemati S, Hassanzadeh R, Khajeh Jahromi S, et al. Otomycosis in the north of Iran: common pathogens and resistance to antifungal agents. Eur Arch Otorhinolaryngol 2014;271:953-7. [Crossref] [PubMed]
- Zhang C, Liu T, Wang Y, et al. Metagenomic next-generation sequencing of bronchoalveolar lavage fluid from children with severe pneumonia in pediatric intensive care unit. Front Cell Infect Microbiol 2023;13:1082925. [Crossref] [PubMed]
- Liu G, Wang L, Li X, et al. The value of next-generation metagenomic sequencing in pathogen detection of pleural effusions and ascites from children with sepsis. Front Cell Infect Microbiol 2023;13:1130483. [Crossref] [PubMed]
- Shen H, Liu T, Shen M, et al. Utilizing metagenomic next-generation sequencing for diagnosis and lung microbiome probing of pediatric pneumonia through bronchoalveolar lavage fluid in pediatric intensive care unit: results from a large real-world cohort. Front Cell Infect Microbiol 2023;13:1200806. [Crossref] [PubMed]
- Zhang Y, Wei E, Niu J, et al. Clinical features of pediatric mucormycosis: role of metagenomic next generation sequencing in diagnosis. Front Cell Infect Microbiol 2024;14:1368165. [Crossref] [PubMed]
- Li FM, Liu J, Yang HQ, et al. The application value of immunofluorescence staining in rapid pathological diagnosis of deep respiratory fungal infections. Experimental and Laboratory Medicine 2024;42:13-6.
- Senécal J, Smyth E, Del Corpo O, et al. Non-invasive diagnosis of Pneumocystis jirovecii pneumonia: a systematic review and meta-analysis. Clin Microbiol Infect 2022;28:23-30. [Crossref] [PubMed]
- Brent AA, Chen O, Eshaq M, et al. Detection of antibody-coated Mucor in skin biopsy by direct immunofluorescence. J Cutan Pathol 2023;50:637-41. [Crossref] [PubMed]
- Zhong S, Yang MH. Value of metagenomic next-generation sequencing in children with hematological malignancies complicated with infections. Chinese Journal of Contemporary Pediatrics 2023;25:718-25. [Crossref] [PubMed]
- Zhu Y, Gan M, Ge M, et al. Diagnostic Performance and Clinical Impact of Metagenomic Next-Generation Sequencing for Pediatric Infectious Diseases. J Clin Microbiol 2023;61:e0011523. [Crossref] [PubMed]
- Liu W, Yang H, Xu Q, et al. Role of MYO1F in neutrophil and macrophage recruitment and pro-inflammatory cytokine production in Aspergillus fumigatus keratitis. Int Immunopharmacol 2024;142:113094. [Crossref] [PubMed]
- Chen J, Yao H, Yuan X, et al. Palatal perforation caused by Alternaria alternata infection in an immunocompetent adolescent. Int J Infect Dis 2023;134:207-10. [Crossref] [PubMed]
- Jing ZC, Chen N, Luo HC. Application of rapid immunofluorescence staining in pulmonary infectious diseases. The Medical Forum 2023;27:101-4, 151.
- Li GR, Bai MM, Tao ZX, et al. A comparative analysis of fluorescent staining and G/GM test of bronchoalveolar lavage fluid in 142 cases. Chinese Journal of Mycology 2021;16:6-9.
- Du E, Pan P, Dou XW. Diagnosis and treatment of fungal infections in the external auditory canal under otoendoscopy: a report of 20 cases. International Infectious Diseases 2020;9:84-5. (Electronic Edition).
- Zhou YD, Zhang F, Xing YR. Efficacy of otoendoscopy in treatment of external auditory canal mycosis. Journal of Medical Forum 2023;44:42-4, 48.
- Li GQ, Xu DY, Zhao FL, et al. Clinical efficacy study of endoscopic cleaning combined with external auditory canal infusion of prednisone in the treatment of pediatric fungal diseases in the external auditory canal. Healthful Friend 2019;(2):62, 42.
- Tai J, Wei YX, Liu C, et al. An external auditory canal medication tool and external auditory canal medication system. China patent: CN202123095403.3. 2022. Available online: https://www.cqvip.com/doc/patent/1966086364?sign=aed4ac5b207cf03b147b49cc54e08badb42b2c9fe817616a1b2ed682b71deec0&expireTime=1751006296384&resourceId=1966086364
- de la Paz Cota BR, Cepero Vega PP, Matus Navarrete JJ, et al. Efficacy and safety of eberconazole 1% otic solution compared to clotrimazole 1% solution in patients with otomycosis. Am J Otolaryngol 2018;39:307-12. [Crossref] [PubMed]
- Swain SK, Behera IC, Sahu MC, et al. Povidone iodine soaked gelfoam for the treatment of recalcitrant otomycosis - Our experiences at a tertiary care teaching hospital of eastern India. J Mycol Med 2018;28:122-7. [Crossref] [PubMed]




