
Fetal tachyarrhythmias: current knowledge for clinical practice
Introduction
Fetal arrhythmias are a significant source of concern for clinicians during routine ultrasound examinations. While fetal arrhythmias are relatively uncommon during pregnancy, their progression can be rapid, leading to fetal death in approximately 10% of cases. Fetal and neonatal arrhythmias with significant hemodynamic impact occur in approximately 1 in 4,000 live births (1). Fetal heart rhythm abnormalities affect approximately 0.5% to 2% of pregnancies and account for 10% to 20% of referrals to specialized centers (2). Tachycardias account for 8% of all fetal arrhythmias (3).
Fetal heart rhythm abnormalities may be associated with structural congenital heart disease (CHD) and may be caused by genetic mutations, myocardial inflammation or ischemia or by transplacental passage of stimulating substances ingested by the mother. The electrocardiogram (ECG) is widely regarded as the gold standard for the postnatal diagnosis of arrhythmias. However, the interference of maternal cardiac activity poses a significant challenge in discerning atrial from ventricular electrical activity in fetuses (3).
Fetal magnetocardiography is a diagnostic method that detects the magnetic fields generated by electrical changes in the fetal heart, enabling the measurement of different intervals in the cardiac cycle. However, it is currently very expensive and only available in a few centers worldwide, limiting its use in routine clinical practice (4,5). Therefore, fetal arrhythmia diagnosis typically relies on mechanical reading of the fetal echocardiogram in M-mode or spectral Doppler. The analysis of atrial and ventricular contractions is a valuable tool in the identification of the underlying mechanism of tachyarrhythmia (6).
There is a lack of studies and evidence-based algorithms for fetal tachyarrhythmia management, leading to considerable variability in treatment approaches depending on local expertise, drug approval, and available resources (7,8).
The purpose of this review is to highlight the physiopathology, diagnosis, classification, and management of the most common fetal tachyarrhythmias.
Embryology and pathophysiology
The electrical stimulus that depolarizes the heart, conducted by the specialized conduction system, comprises the sinus node (SN) itself, the intranodal tracts, the Bachmann bundle, the atrioventricular (AV) node, the His bundle, the right and left branches with their fascicles, and the Purkinje fibers. The role of this system is to generate and propagate the electrical impulse through the myocardium to produce each heartbeat. The SN is situated at the intersection of the right common cardinal vein and the right atrium’s wall, within the terminal groove, and is morphologically identifiable at approximately 35 days of life (9,10). The AV node is formed separately from the bundle of His in the 10th week of gestation, and the union of the two is secondary. The conduction system of the fetal heart is functionally mature at about 16 weeks of gestation (11-13).
The expression of the transcription factor Tbx3 is required for the development of a normal SN. Pathogenic variants in specific genes are associated with the development of transcription factors (TBX5, TBX3 and SHOX2) which in turn predispose individuals to SN disease (12,14), contingent on their epigenetic and transcriptional state (15). Indeed, Tbx2 contributes to the maturation of the AV node conduction system and may be related to genes in Wolf-Parkison-White (WPW) tachyarrhythmias (16). The knowledge of these molecular pathways provides us a better understanding of the abnormal development of the cardiac conduction system.
The SN is a set of specialized autonomous cells that function as the heart’s intrinsic pacemaker, located near the mouth of the superior vena cava in the right atrium. The electrical stimulus from the SN is propagated by contractile atrial cells arranged in bundles in the right atrium and to the left atrium by nodal myocardial bands known as Bachmann’s bundle, a fast pathway located anterosuperiorly connecting the two atrial appendages. Another pathway lies inferiorly near the coronary sinus and pulmonary veins (17,18).
In normal hearts, the electrical impulse generated by the SN travels through the atrial myocardium to the AV node, which is located in Koch’s triangle. The definition of this triangle is extremely important because it contains the AV node and the initial portion of the His bundle. The cells that make up the AV node are grouped in the region near the apex of Koch’s triangle. They are specialized cardiomyocytes with a smaller diameter and fewer myofilaments than the contractile cells. The AV node is responsible for delaying the AV conduction before it is conducted to the ventricles via the His and Purkinje fibers (13).
All the structures specialized for the conduction of electrical stimuli may present anomalies, intrinsic or not, and may be associated with structural cardiac changes. Normal electrical conduction uses anterograde conduction of the AV nodal pathway. Abnormal conduction of the electrical stimulus through the accessory pathways may use anterograde AV conduction, retrograde conduction or both (13).
Diagnosis
Fetal magnetocardiography is a non-invasive technique for recording the electrical activity of the fetal heart, analogous to an ECG (19). This technique elucidates the mechanisms of tachyarrhythmias and even identifies long QT syndrome, but it is a very expensive method and very few centers have this technology (20-22).
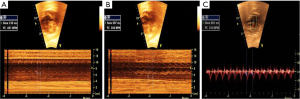
Cardiac rhythm can also be interpreted non-invasively and inexpensively using ultrasound to observe the movements and currents caused by atrial and ventricular contractions during systole. Atrial systole corresponds to the A wave on Doppler of the AV valves, by conventional or tissue Doppler, and on venous Doppler (pulmonary vein, vena cava and ductus venosus) and via M-mode by the contraction of the atrial wall. Ventricular systole is identified by Doppler of the aortic or pulmonary flow, or by the systolic movement of the ventricular wall in M-mode (Figures 1,2) (13,23-25).
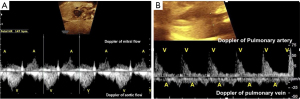
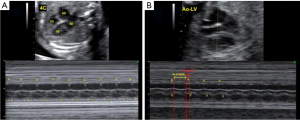
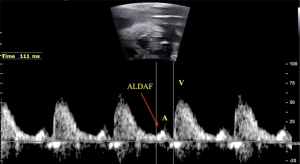
Several factors can complicate heart rhythm assessment, including first trimester evaluation, unfavorable fetal position, hydropic fetuses with cardiac contractile dysfunction, and obese pregnant women. In such cases, the use of anatomic M-mode (AMM) and the assessment of anterograde late diastolic arterial blood flow (ALDAF) are effective methods for evaluating cardiac rhythm. The AMM provides the M-mode assessment of the mechanical movements of the atrial and ventricular walls independent of the transducer orientation (26). Similarly, the assessment of retrograde A and S waves via Doppler of aortic or pulmonary arterial flow facilitates AV conduction evaluation, especially if the heart position is not apical (Figure 3) (13,27-29).
In the presence of fetal cardiac tachyarrhythmias, the following should always be assessed:
- Ventricular rate (VR)—fetal tachycardia is defined as a fetal heart rate (HR) >180 bpm.
- Rhythm frequency and regularity—the tachycardia duration is rare when occurs <10% of time during monitoring, is intermittent when present for 10–50% of the monitoring time (exam time) and sustained or incessant when present for ≥50% of echocardiographic monitoring time (2).
- AV ratio—if atrial and ventricular HR (AV ratio 1:1) are similar, if atrial HR is higher than ventricular HR or if ventricular HR is higher than atrial HR.
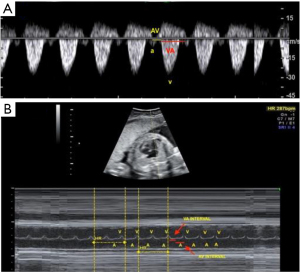
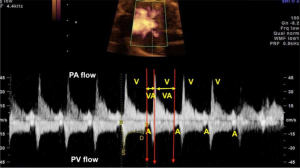
- AV and ventricular-atrial (VA) intervals—obtained by real-time two-dimensional (2D) imaging with the M-mode beam passed simultaneously through the atrial and ventricular walls, or by Doppler imaging of mitral-aortic flow or superior vena cava—aorta. Doppler measures the AV interval from the start of the atrial (A) wave to the start of the ventricular (V) wave. The VA interval is the interval between the beginning of the V wave and the A wave. The M-mode is used to assess the movements of the A and V walls. The AV and VA intervals are mechanical analogues of the electrical PR and RP intervals on the ECG (23). These intervals are particularly important for analyzing AV conduction (13,30). Ratio of VA to AV intervals (8). VA/AV ratio >1 long AV tachycardia (ectopic atrial tachycardia, permanent junctional reciprocating tachycardia, sinus tachycardia (ST), nodal re-entry tachycardia). VA/AV ratio <1 short AV tachycardia (AV re-entry tachycardia) (Figure 4).
Fetal heart function
The fetal myocardium has a reduced contractile capacity due to myocardial immaturity, reduced compliance, dependence of cardiac output on fetal HR and reduced sympathetic innervation. These characteristics make the fetal heart highly susceptible to heart failure (HF) in response to sustained tachycardia, potentially leading to hydrops, multiple organ failure, and fetal death (31-33).
For the echocardiographic assessment of fetal heart function (21,34), a cardiovascular profile score (CPS) has been proposed to evaluate the presence and severity of HF. This score uses five ultrasound parameters: (I) fetal effusions (hydrops); (II) venous Doppler; (III) cardiac size; (IV) cardiac function; (V) arterial Doppler. A score of 10 is considered normal. Scores 8–9= mild HF, 6–7= moderate HF and <5= severe HF. CPS <7 require intervention and indicates a more severe fetal HF with a higher risk of adverse outcomes. The CPS can help predict fetal outcome, guide management decisions, and potentially improve perinatal outcomes (30,31).
Classification of tachyarrhythmias
Fetal tachyarrhythmias can be divided into ST, supraventricular tachycardia (SVT) and ventricular tachycardia (VT). The most common type is SVT, accounting for approximately 70–75% of cases (13,35), though some studies report incidences as high as 90% (8,36). SVT includes tachycardias originating in the atria or AV node and the risk of progression to hydrops is 30–40%. Sustained fetal tachycardia can result in Ballantyne syndrome, a maternal complication characterized by fetal hydrops.
ST
In the ST, the fetal HR is elevated but maintains a regular rhythm with 1:1 conduction, meaning atrial and ventricular frequencies are similar, though variable, and typically below 200 bpm (13). Between 160 and 180 bpm, probable causes of ST should also be investigated. The causes related to this increase in fetal HR are fetal agitation, maternal stress, the maternal use of β-receptor agonists or stimulants (food or drink), infections (cytomegalovirus, chorioamnionitis among others) or conditions such as anemia, fetal distress and hyperthyroidism, especially in incessant tachycardia (8,37).
The initial approach involves identifying and addressing the underlying cause through laboratory tests and eliminating any contributing agents. Weekly follow-ups to monitor HR are usually sufficient. Specific antiarrhythmic therapy is not indicated, but the underlying condition must be managed (1,2,21).
SVT
Atrial flutter
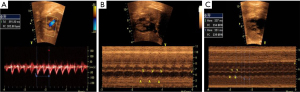
Up to 25% of fetal tachyarrhythmias may be atrial. Atrial HR is caused by a regular and rapid electrical impulse that rotates around the atrium and is self-sustaining (macroreentrant circuit), being more common in mid and late gestation (8,22). Ventricular HR is slower due to the refractory nature of the AV node, which prevents some of the atrial impulses from reaching the ventricles. Atrial HR usually varies between 300–500 bpm. AV conduction is usually 2:1 or 4:1, with ventricular HR 2 or 3 times lower than atrial HR, regular but can be irregular (Figure 5) (38). A case of fetus atrial flutter with 1:1 AV conduction and an HR over 300 bpm has been described by Leiria et al. (39). Atrial flutter is associated with structural cardiac defects and maternal autoimmune disease (anti-Ro/SSA) and or anti-La/SSB autoantibodies with a risk of 10% to 15% to develop HF (8,21,37,39-41). In the absence of cardiac defects, postnatal recurrence of atrial flutter is a rarely observed occurrence, irrespective of the utilization of preventive treatment (42,43).
Ectopic atrial tachycardia
Ectopic atrial tachycardia is caused by impulses from several ectopic sites (in and around the atria) in a disorganized way. These signals are usually chaotic and cause the atria to quiver rather than contract. Most of the atrial electrical impulses do not reach the ventricles because of the refractory properties of the AV node. The atrial HR is extremely high, and the impulses that pass through the AV node are irregular. Atrial tachycardia is very rare in the fetus and is observed in the last weeks of pregnancy and can be associated with Costello’s syndrome. Polyhydramnios with nuchal thickening, hydrops, shortened long bones, abnormal hand posture, ventriculomegaly, macrocephaly, and especially fetal atrial tachycardia are important features of Costello’s syndrome (8,43). The atrial rate varies between 180 and 220 bpm, with 1:1 conduction. These tachycardias are difficult to treat, being that the treatment is recommended when the mean HR is >200 bpm, or >160 and <200 bpm with hydrops (8,21).
Supraventricular re-entry tachycardia
Supraventricular re-entry tachycardia is the most common type of tachycardia in the fetus. It is caused by a re-entrant circuit in which the AV node conducts the impulse anterogradely from the atria to the ventricles, and a fast accessory pathway conducts the ventricular impulse back to the atria. On the other hand, non-conduction of the impulse occurs when it reaches non-excitable tissue, either because it is still refractory after a recent depolarization (blocked premature atrial contraction) or because of abnormal tissue (heart block) (8). Atrial and ventricular HR are identical, with HR of 220 to 300 bpm with 1:1 conduction and VA/AV ratio <1 (short VA interval) (Figure 6). Most hearts are structurally normal, but Ebstein’s anomaly is known to be associated with accessory pathways (Figure 7). During the coronavirus disease 2019 (COVID-19) pandemic, some studies have demonstrated a significant increase in the incidence of SVT, probably as a result of fetal hypoxia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection of the placenta (44,45).
Some authors recommend close monitoring of fetuses with supraventricular re-entry tachycardia. In the case of non-sustained, brief, or infrequent episodes, maintenance without drug therapy may be a safe approach, as HF rarely develops unless the HR is very rapid and/or becomes more persistent. In persistent or incessant HF, hydrops is common and is strongly associated with fetal death (46). With drug therapy and normalization of the heart rhythm, fetal hydrops resolves over time. Medications include digoxin, flecainide, or maternal sotalol, alone or in combination, while amiodarone and direct fetal therapy are generally reserved for treatment-resistant and poorly tolerated tachycardia (8). The risk of recurrence of SVT appears to be 50% in cases of fetal SVT. Transesophageal programmed stimulation is a possible method to further evaluate the risk of recurrence (8,47,48).
Ectopic atrial tachycardia
Ectopic atrial tachycardia is a rare form of tachycardia caused by an ectopic focus within the atria that takes over the pacemaker function of the heart and is usually sustained. Ectopic atrial tachycardia is characterized by intermittent changes with gradual onset and termination. Conduction is usually 1:1, with a VA/AV ratio >1, conduction delay with AV block may be observed, being classified as a long VA SVT. Similar to the management of supraventricular re-entry tachycardia, postnatal antiarrhythmic treatment is commonly required (8,42).
Ventricular arrythmias
VT
VT is rare in the fetal period, accounting for <2% of all tachyarrhythmias (6,8). The VR is between 180 and 300 bpm, with a VA/AV ratio <1. The mechanism involved is ectopic focus in the ventricle, usually caused by inflammation or abnormal myocardial oxygen delivery. VT can occur in fetuses with positive AV block and anti-Ro/SSA antibody, cardiac tumors, viral or hereditary cardiomyopathy, ion channelopathies such as long QT syndrome, structural heart disease such as ventricular aneurysm, and electrolyte imbalance (49,50).
Long QT
Long QT syndrome is an autosomal dominant syndrome that can present as isolated mild bradycardia, 2nd degree AV block, or VT such as torsades de pointes (50). This syndrome should be considered primarily in the setting of sinus bradycardia or 2nd degree AV block and VT (37). Fetal prolonged QT can be confirmed using fetal magnetocardiography. Management includes careful observation and evaluation for a family history of long QT syndrome. Regardless of family history, an ECG of the mother and partner should be obtained.
Maternal electrolyte abnormalities, hypomagnesemia, and hypocalcemia, as well as drugs and anesthetics with QT interval prolongation potential, should be avoided (5). Fetal treatment is recommended for VT, such as torsades de pointes, in early or mid-gestation cases. Preparations for labor may include a planned delivery with a pediatric cardiologist, medications such as intravenous magnesium on hand, and a neonatal defibrillator/pacemaker and resuscitation cart readily available (49-51).
Treatment
The management of fetal tachycardia depends on several factors, including gestational age, degree of fetal involvement, associated risk factors, the clinical condition of the mother, and the potential maternal risks associated with treating the fetus. The decision to anticipate delivery—given the complications of prematurity—must be carefully balanced against available therapies, their efficacy, and the risks to both maternal and fetal health. All fetuses with persistent tachycardia, as well as those exhibiting intermittent tachycardia with ventricular dysfunction and/or fetal hydrops, should receive pharmacologic treatment, unless the pregnancy is near term, which carries a low risk of early delivery (8,21).
The routes of administration for antiarrhythmic drugs can vary: transplacental, umbilical vein injection, fetal intra-abdominal, amniotic fluid, and fetal intramuscular injections (36). Transplacental therapy is the most widely used, although it exposes the mother to the side effects and risks of antiarrhythmic drugs (52). The other routes are limited due to their invasive nature and are typically only used when transplacental transfer is low (8). Fetal transesophageal pacing was first described by Stirnemann et al. (53) and later by Wang et al. (54), both of whom reported successful outcomes in two cases of severe, refractory fetal tachycardia in fetuses at 27+5 and 26+2 weeks of gestation, respectively. However, due to the significant risks associated with the procedure, including premature rupture of ovular membranes and atrial fibrillation, this therapy is currently considered a last-resort option at experienced medical centers. The goal of treatment is to suppress the arrhythmia or, if that is not achievable, to reduce the VR to a more normal HR. Prenatal treatment primarily involves drug therapy, with medications such as digoxin, flecainide, sotalol, and other clinically supported drugs being used as first-line treatments (1,21,49). Digoxin is the longest-used first-line drug for treating fetal tachycardias, particularly those with a VA/AV ratio >1, such as supraventricular re-entry tachycardia. However, its ability to cross the edematous placenta is limited, necessitating higher and more frequent doses to reach therapeutic levels in the fetus (41,42). In such cases, additional medications, such as a combination of digoxin with sotalol or flecainide, are required (41,54). Additionally, many studies have shown that the reversal rate of fetal tachycardias with a long VA interval is poor (23,49).
Flecainide and sotalol are considered superior to digoxin in treating SVT with or without fetal edema, according to two meta-analyses (33,55). This difference is particularly evident when fetal edema is present or in cases of SVT with a long VA interval, where flecainide’s efficacy surpasses that of digoxin (8,56). While there is no randomized clinical trial demonstrating the superiority of one antiarrhythmic drug over another, flecainide and sotalol (rather than digoxin) are increasingly becoming the first-line treatments for fetal SVT and atrial flutter. Some studies have suggested that fetuses treated with antiarrhythmic drugs experience reduced rates of premature birth and neonatal arrhythmias (2,22,57,58). In 2017, the International Fetal Medicine and Surgery Society (IFMSS), in collaboration with the North American Fetal Therapy Network (NAFTNet), emphasized that the goal of fetal therapy has expanded beyond merely improving fetal survival to also focusing on maternal health and safety, with the aim of minimizing complications (59). The initial maternal evaluation includes a thorough history, physical examination, ECG, and echocardiogram. When antiarrhythmic medications are administered, it is crucial to monitor the mother closely to minimize potential side effects (49). The therapy for atrial flutter and SVT is the subject of an ongoing prospective, multicenter phase 3 study. While the study investigates antiarrhythmic therapies and their indications, it highlights a significant gap in evidence regarding the efficacy and safety of these drugs and their effects on both the mother and fetus for optimal management. As a result, physicians are often required to make management decisions without the benefit of controlled trial data (59). Recent studies indicate superior results for flecainide or sotalol compared to digoxin as first-line treatments for SVT or atrial flutter. In supraventricular re-entry tachycardia, flecainide has been shown to be a more effective first-line treatment than both digoxin and sotalol (8,60). Studies also demonstrate that flecainide is superior in treating SVT, with success rates of 90% to 96% in cases without hydrops and 66% to 86% in cases with hydrops (8,33,61). However, it is important to note that flecainide is not commercially available for use in all countries. A comprehensive systematic review and meta-analysis evaluating the efficacy of digoxin, flecainide, and sotalol in converting fetal SVT (from 21 trials) found that flecainide was superior to digoxin in converting fetuses with and without hydrops to sinus rhythm. When compared with digoxin, sotalol was found to be superior in fetuses with hydrops, while no significant difference was found in fetuses without hydrops. No significant difference was observed between flecainide and sotalol in fetuses with or without hydrops (33,60). Regarding VT, one report described rhythm reversal after the pregnant woman was hospitalized and treated with a combination of flecainide and magnesium sulfate. The finding of variable AV block after conversion from VT provides evidence of long QT (60).
The main therapeutic schemes recommended by the American Heart Association, the Barcelona Fetal Medicine Group, and various pediatric and congenital electrophysiology societies for the treatment of fetal supraventricular re-entry tachyarrhythmia and atrial flutter are illustrated in Figures 8-10 (1,2). When available, flecainide can be used alone as a first-line treatment for SVT without hydrops or in combination with digoxin when hydrops is present (Figure 11). Figure 11 provides a concise overview of the recommended dosages for the most prescribed medications used in the management of fetal tachyarrhythmias.
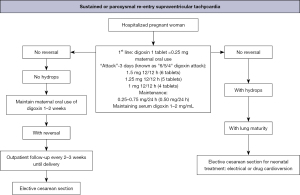
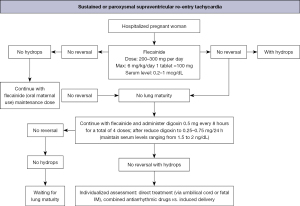
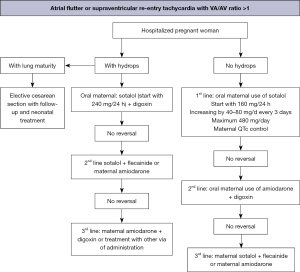
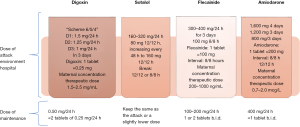
Amiodarone has a more significant toxicity profile in pregnant women and fetuses and is recommended as a third-line treatment for SVT. After flecainide and sotalol, amiodarone may also be considered for atrial flutter. For sustained VT, first-line treatment is maternal intravenous magnesium sulfate when fetal HR exceeds 200 bpm. Due to its short duration of action (<48 hours), additional medications such as intravenous lidocaine, oral propranolol, or oral mexiletine may be added. Flecainide, sotalol, and amiodarone should be avoided if long-QT syndrome is suspected (35,42,62-64). If the VT is related to isoimmunization or myocarditis, the use of dexamethasone and intravenous immunoglobulin may be considered (21,49).
Conclusions
The potential for fetal treatment to improve prognoses in both fetal and postnatal life has been well demonstrated. A variety of treatment options exist for fetal tachyarrhythmias, with the most effective drug selected based on the expertise of the healthcare team and the practical considerations of the treatment. However, a lack of consensus remains, particularly regarding the long-term implications of medication use for both pregnant women and fetuses. It is crucial to emphasize that the initiation of treatment should be guided by the persistence of fetal tachycardia, with careful consideration given to the balance between the risks of premature birth and the potential adverse effects of pharmaceutical interventions on both the mother and the fetus.
Acknowledgments
None.
Footnote
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-67/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-67/coif). E.A.J. serves as an unpaid editorial board member of Translational Pediatrics from October 2023 to September 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Batra AS, Silka MJ, Borquez A, et al. Pharmacological Management of Cardiac Arrhythmias in the Fetal and Neonatal Periods: A Scientific Statement From the American Heart Association: Endorsed by the Pediatric & Congenital Electrophysiology Society (PACES). Circulation 2024;149:e937-52. Erratum in: Circulation 2024;149:e996. [Crossref] [PubMed]
- Pedra SRFF, Zielinsky P, Binotto CN, et al. Brazilian Fetal Cardiology Guidelines - 2019. Arq Bras Cardiol 2019;112:600-48. [Crossref] [PubMed]
- Nogué L, Gómez O, Masoller N, et al. Fetal Arrhythmias. Available online: https://fetalmedicinebarcelona.org/wp-content/uploads/2024/08/arritmias_en.pdf
- Carvalho JS. Fetal dysrhythmias. Best Pract Res Clin Obstet Gynaecol 2019;58:28-41. [Crossref] [PubMed]
- Strasburger JF, Cheulkar B, Wakai RT. Magnetocardiography for fetal arrhythmias. Heart Rhythm 2008;5:1073-6. [Crossref] [PubMed]
- Strand SA, Strasburger JF, Wakai RT. Fetal magnetocardiogram waveform characteristics. Physiol Meas 2019;40:035002. [Crossref] [PubMed]
- Lopes LM, Zugaib M. Fetal arrhythmias. Arq Bras Cardiol 1997;69:219-22. [Crossref] [PubMed]
- Alsaied T, Baskar S, Fares M, et al. First-Line Antiarrhythmic Transplacental Treatment for Fetal Tachyarrhythmia: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2017;6:e007164. [Crossref] [PubMed]
- Tang J, Huang P, Deng X, et al. Advances and challenges of prenatal interventions for fetal tachyarrhythmias. Front Pediatr 2024;12:1509158. [Crossref] [PubMed]
- Bhattacharyya S, Munshi NV. Development of the Cardiac Conduction System. Cold Spring Harb Perspect Biol 2020;12:a037408. [Crossref] [PubMed]
- van der Maarel LE, Christoffels VM. Development of the Cardiac Conduction System. Adv Exp Med Biol 2024;1441:185-200. [Crossref] [PubMed]
- Munshi NV. Gene regulatory networks in cardiac conduction system development. Circ Res 2012;110:1525-37. [Crossref] [PubMed]
- van Weerd JH, Christoffels VM. The formation and function of the cardiac conduction system. Development 2016;143:197-210. [Crossref] [PubMed]
- Bravo-Valenzuela NJ, Rocha LA, Machado Nardozza LM, et al. Fetal cardiac arrhythmias: Current evidence. Ann Pediatr Cardiol 2018;11:148-63. [Crossref] [PubMed]
- Basalamah F, Dilogo IH, Raharjo SB, et al. TBX3 transfection and nodal signal pathway inhibition promote differentiation of adipose mesenchymal stem cell to cardiac pacemaker-like cells. Stem Cell Res Ther 2024;15:148. [Crossref] [PubMed]
- van der Maarel LE, Postma AV, Christoffels VM. Genetics of sinoatrial node function and heart rate disorders. Dis Model Mech 2023;16:dmm050101. [Crossref] [PubMed]
- Hatcher CJ, Basson CT. Specification of the cardiac conduction system by transcription factors. Circ Res 2009;105:620-30. [Crossref] [PubMed]
- Kashou AH, Basit H, Chhabra L. Physiology, Sinoatrial Node. Treasure Island, FL, USA: StatPearls Publishing; 2025.
- Lang D, Glukhov AV. Functional Microdomains in Heart's Pacemaker: A Step Beyond Classical Electrophysiology and Remodeling. Front Physiol 2018;9:1686. [Crossref] [PubMed]
- Zhao H, Strasburger JF, Cuneo BF, et al. Fetal cardiac repolarization abnormalities. Am J Cardiol 2006;98:491-6. [Crossref] [PubMed]
- Crowe JA, Herbert JM, Huang XB, et al. Sequential recording of the abdominal fetal electrocardiogram and magnetocardiogram. Physiol Meas 1995;16:43-7. [Crossref] [PubMed]
- Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation 2014;129:2183-242. [Crossref] [PubMed]
- Wacker-Gussmann A, Strasburger JF, Wakai RT. Contribution of Fetal Magnetocardiography to Diagnosis, Risk Assessment, and Treatment of Fetal Arrhythmia. J Am Heart Assoc 2022;11:e025224. [Crossref] [PubMed]
- Jaeggi E, Fouron JC, Fournier A, et al. Ventriculo-atrial time interval measured on M mode echocardiography: a determining element in diagnosis, treatment, and prognosis of fetal supraventricular tachycardia. Heart 1998;79:582-7. [Crossref] [PubMed]
- Nii M, Hamilton RM, Fenwick L, et al. Assessment of fetal atrioventricular time intervals by tissue Doppler and pulse Doppler echocardiography: normal values and correlation with fetal electrocardiography. Heart 2006;92:1831-7. [Crossref] [PubMed]
- Anuwutnavin S, Kolakarnprasert K, Chanprapaph P, et al. Measurement of fetal atrioventricular time intervals: A comparison of 3 spectral Doppler techniques. Prenat Diagn 2018;38:459-66. [Crossref] [PubMed]
- Jürgens J, Chaoui R. Three-dimensional multiplanar time-motion ultrasound or anatomical M-mode of the fetal heart: a new technique in fetal echocardiography. Ultrasound Obstet Gynecol 2003;21:119-23. [Crossref] [PubMed]
- Azeka E, Jatene MB, Jatene IB, et al. I Guidelines of heart failure and heart transplantation in the fetus, in children and adults with congenital cardiopathy, The Brazilian Society of Cardiology. Arq Bras Cardiol 2014;103:1-126. Erratum in: Arq Bras Cardiol 2016;106:267. [Crossref] [PubMed]
- Howley LW, Yamamoto Y, Sonesson SE, et al. Antegrade late diastolic arterial blood flow in the fetus: insight into fetal atrial function. Am J Obstet Gynecol 2013;208:490.e1-8. [Crossref] [PubMed]
- Heetchuay T, Trakulmungkichkarn T, Pabalan N, et al. Reference values of fetal atrioventricular time intervals derive from antegrade late diastolic arterial blood flow (ALDAF) from 14 to 40 weeks of gestation. Clin Exp Obstet Gynecol 2021;48:867-74.
- Mosimann B, Arampatzis G, Amylidi-Mohr S, et al. Reference Ranges for Fetal Atrioventricular and Ventriculoatrial Time Intervals and Their Ratios during Normal Pregnancy. Fetal Diagn Ther 2018;44:228-35. [Crossref] [PubMed]
- Huhta JC. Fetal congestive heart failure. Semin Fetal Neonatal Med 2005;10:542-52. [Crossref] [PubMed]
- Hofstaetter C, Hansmann M, Eik-Nes SH, et al. A cardiovascular profile score in the surveillance of fetal hydrops. J Matern Fetal Neonatal Med 2006;19:407-13. [Crossref] [PubMed]
- Hill GD, Kovach JR, Saudek DE, et al. Transplacental treatment of fetal tachycardia: A systematic review and meta-analysis. Prenat Diagn 2017;37:1076-83. [Crossref] [PubMed]
- Moon-Grady AJ, Donofrio MT, Gelehrter S, et al. Guidelines and Recommendations for Performance of the Fetal Echocardiogram: An Update from the American Society of Echocardiography. J Am Soc Echocardiogr 2023;36:679-723. [Crossref] [PubMed]
- Krapp M, Kohl T, Simpson JM, et al. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart 2003;89:913-7. [Crossref] [PubMed]
- Pézard PG, Boussion F, Sentilhes L, et al. Fetal tachycardia: a role for amiodarone as first- or second-line therapy? Arch Cardiovasc Dis 2008;101:619-27. [Crossref] [PubMed]
- Veduta A, Panaitescu AM, Ciobanu AM, et al. Treatment of Fetal Arrhythmias. J Clin Med 2021;10:2510. [Crossref] [PubMed]
- Leiria TL, Lima GG, Dillenburg RF, et al. Fetal tachyarrhythmia with 1:1 atrioventricular conduction. Adenosine infusion in the umbilical vein as a diagnostic test. Arq Bras Cardiol 2000;75:65-8. [Crossref] [PubMed]
- Wacker-Gussmann A, Strasburger JF, Srinivasan S, et al. Fetal Atrial Flutter: Electrophysiology and Associations With Rhythms Involving an Accessory Pathway. J Am Heart Assoc 2016;5:e003673. [Crossref] [PubMed]
- Holmes S, Hornberger LK, Jaeggi E, et al. Treatment, not delivery, of the late preterm and term fetus with supraventricular arrhythmia. Ultrasound Obstet Gynecol 2023;62:552-7. [Crossref] [PubMed]
- Miyoshi T, Maeno Y, Hamasaki T, et al. Antenatal Therapy for Fetal Supraventricular Tachyarrhythmias: Multicenter Trial. J Am Coll Cardiol 2019;74:874-85. [Crossref] [PubMed]
- Jaeggi ET, Carvalho JS, De Groot E, et al. Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: results of a nonrandomized multicenter study. Circulation 2011;124:1747-54. [Crossref] [PubMed]
- Lin AE, O'Brien B, Demmer LA, et al. Prenatal features of Costello syndrome: ultrasonographic findings and atrial tachycardia. Prenat Diagn 2009;29:682-90. [Crossref] [PubMed]
- Samples S, Patel S, Lee S, et al. Incidence of Fetal Arrhythmia Before and During the COVID-19 Pandemic: A Single-Center Experience. Pediatr Cardiol 2025;46:431-6. [Crossref] [PubMed]
- Mullins J, Bewley DJ, Oviedo A. COVID-19 and Placental Infection: Are Fetal Survivors at Risk of Long-Term Cardiovascular Complications? Cureus 2023;15:e38077. [Crossref] [PubMed]
- Simpson JM, Sharland GK. Fetal tachycardias: management and outcome of 127 consecutive cases. Heart 1998;79:576-81.
- Michel M, Renaud C, Chiu-Man C, et al. Postnatal recurrence and transesophageal inducibility of prenatally treated fetal supraventricular tachycardia. Heart Rhythm 2022;19:1343-9. [Crossref] [PubMed]
- Jaeggi E, Öhman A. Fetal and Neonatal Arrhythmias. Clin Perinatol 2016;43:99-112. [Crossref] [PubMed]
- Joglar JA, Kapa S, Saarel EV, et al. 2023 HRS expert consensus statement on the management of arrhythmias during pregnancy. Heart Rhythm 2023;20:e175-264. [Crossref] [PubMed]
- Mitchell JL, Cuneo BF, Etheridge SP, et al. Fetal heart rate predictors of long QT syndrome. Circulation 2012;126:2688-95. [Crossref] [PubMed]
- Chimenea Á, Vargas-Rodríguez C, García-Díaz L, et al. Transplacental Treatment of Fetal Tachyarrhythmia: Current Trends and Future Perspectives. Future Pharmacology 2023;3:440-50.
- Stirnemann J, Maltret A, Haydar A, et al. Successful in utero transesophageal pacing for severe drug-resistant tachyarrhythmia. Am J Obstet Gynecol 2018;219:320-5. [Crossref] [PubMed]
- Wang H, Luo W, Gongli C. Fetal Therapy for Severe Drug-Resisted Tachyarrhythmia With Progressive Hydrops by Fetoscopic Transesophageal Pacing: A Successful Attempt in Single Chinese Fetal Medicine Center. Prenat Diagn 2025;45:652-5. [Crossref] [PubMed]
- Yuan SM, Xu ZY. Fetal arrhythmias: prenatal evaluation and intrauterine therapeutics. Ital J Pediatr 2020;46:21. [Crossref] [PubMed]
- Sridharan S, Sullivan I, Tomek V, et al. Flecainide versus digoxin for fetal supraventricular tachycardia: Comparison of two drug treatment protocols. Heart Rhythm 2016;13:1913-9. [Crossref] [PubMed]
- Qin J, Deng Z, Tang C, et al. Efficacy and Safety of Various First-Line Therapeutic Strategies for Fetal Tachycardias: A Network Meta-Analysis and Systematic Review. Front Pharmacol 2022;13:935455. [Crossref] [PubMed]
- Ueda K, Maeno Y, Miyoshi T, et al. The impact of intrauterine treatment on fetal tachycardia: a nationwide survey in Japan. J Matern Fetal Neonatal Med 2018;31:2605-10. [Crossref] [PubMed]
- Moon-Grady AJ, Baschat A, Cass D, et al. Fetal Treatment 2017: The Evolution of Fetal Therapy Centers - A Joint Opinion from the International Fetal Medicine and Surgical Society (IFMSS) and the North American Fetal Therapy Network (NAFTNet). Fetal Diagn Ther 2017;42:241-8. [Crossref] [PubMed]
- Jaeggi E. FAST RCT: Prospective Randomized Clinical Trial of Fetal Atrial Flutter & Supraventricular Tachycardia Therapy. 2016-2024. Available online: https://clinicaltrials.ucsf.edu/trial/NCT02624765
- Simpson JM, Maxwell D, Rosenthal E, et al. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: case report and review of the literature. Ultrasound Obstet Gynecol 2009;34:475-80. [Crossref] [PubMed]
- Strizek B, Berg C, Gottschalk I, et al. High-dose flecainide is the most effective treatment of fetal supraventricular tachycardia. Heart Rhythm 2016;13:1283-8. [Crossref] [PubMed]
- Shah A, Moon-Grady A, Bhogal N, et al. Effectiveness of sotalol as first-line therapy for fetal supraventricular tachyarrhythmias. Am J Cardiol 2012;109:1614-8. [Crossref] [PubMed]
- Strasburger JF, Cuneo BF, Michon MM, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation 2004;109:375-9. [Crossref] [PubMed]


