
PPP1R12A mutation leads to different genders of twinning: a case report and literature review
Highlight box
Key findings
• A rare de novo variant (c.1551-2A>G) in protein phosphatase 1 regulatory subunit 12A (PPP1R12A) was identified in a pair of monozygotic twins presenting with urogenital and/or brain malformation syndrome (UBMS) and disorders of sex development (DSD). The twins exhibited distinct genital phenotypes and were assigned different genders.
What is known and what is new?
• Loss-of-function variants in PPP1R12A are associated with UBMS. When UBMS is accompanied by genital abnormalities, it is often linked to DSD. To date, 18 de novo loss-of-function variants in the PPP1R12A gene have been reported.
• This study reports the first case of monozygotic twins with the same PPP1R12A mutation (c.1551-2A>G) exhibiting divergent phenotypes. Our findings provide valuable clinical insights into gender assignment and management strategies for UBMS-associated DSD cases.
What is the implication, and what should change now?
• In cases of UBMS with DSD, a comprehensive evaluation of internal genitalia and gonadal function is crucial for informed gender assignment. Management by a multidisciplinary team ensures tailored care, while timely gender assignment and long-term follow-up are essential to optimize outcomes and support patient well-being.
Introduction
Protein phosphatase 1 regulatory subunit 12A (PPP1R12A [MIM:602021]) is a 161.9-kb-long gene containing 25 exons located on chromosome 12q21.2-q21.31, and its protein product (PPP1R12A) is a component of myosin phosphatase (MP), an essential enzyme for smooth muscle contraction and is involved in diverse and unexpected functions (1). To date, only 18 de novo loss-of-function variants in the PPP1R12A gene have been identified in 18 individuals. (Human Gene Mutation Database: http://www.hgmd.cf.ac.uk/). These PPP1R12A variant carriers are linked to urogenital and/or brain malformation syndrome (UBMS, OMMI#3618820), which is characterized by a number of congenital abnormalities, most often urogenital malformations, developmental delay and brain malformations (2). Individuals with a 46,XY karyotype often present with a uterus and varying degrees of under-masculinization, associated with disorders of sex development (DSD) (2,3). Overall, PPP1R12A-related UBMS and DSD are rare, no associations between genotype and phenotype have been found, and the experience of treatment is also rarely reported (2-4). Here, we report a case of identical twins who had 46,XY DSD, and UBMS due to a novel de novo mutation in PPP1R12A. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-166/rc).
Case presentation
Medical history
Twin A, a 10-month-old child raised as a boy, was brought to our clinic in May 2022 due to ambiguous external genitalia. Twin B exhibited similar clinical features, including severe hypospadias, left cryptorchidism and right hernia. Twin B had previously undergone stage I hypospadias repair at a local hospital. Both twins were monozygotic, conceived spontaneously, and delivered via cesarean section at 37 weeks following an uncomplicated pregnancy. No family history of developmental diseases or other congenital abnormalities was noted.
Physical examination
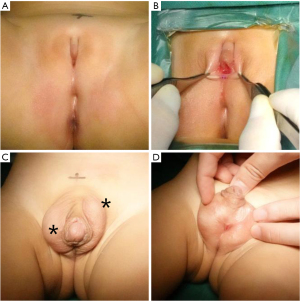
Both twins had height and weight within normal limits for age. Twin A exhibited Prader stage III genitalia (Figure 1A,1B), characterized by a markedly small penis resembling a clitoris, a single urogenital sinus opening in the perineum, and nonpalpable gonads. The external masculinization score (EMS) for Twin A was 0 (normal male score: 12) (5). Twin B presented with more masculine external genitalia (Prader stage IV) (Figure 1C,1D) and an EMS of 5.5. Twin B’s penis was well-developed, with testes-like masses palpable in the left inguinal region and right scrotum, and a single urogenital sinus opening in the scrotum.
Laboratory examinations (Table 1)
Table 1
| Presentation | Twin A | Twin B | |||
|---|---|---|---|---|---|
| Pre-HCG | Post-HCG | Pre-HCG | Post-HCG | ||
| LH (IU/L) (NR: <0.4) | 26.23 | 14.54 | 0.95 | 0.11 | |
| FSH (IU/L) (NR: 0.5–3.2) | 140.22 | 111.50 | 4.10 | 1.42 | |
| Estradiol (pmol/L) (NR: <160.3) | <36.70 | 42.50 | <36.70 | <36.70 | |
| Total T (nmol/L) (NR: <0.7) | <0.45 | <0.45 | <0.45 | 13.28 | |
| DHT (pg/mL) (NR: 23.5–116) | 87.71 | 50.24 | 11.29 | 191.50 | |
| AMH (ng/mL) (NR: >23) | 0.02 | – | >23 | – | |
| Inhibin B (pg/mL) (NR: 50–180) | 10 | – | 145 | – | |
| 17α-OHP (nmol/L) (NR: 0–11.5) | 0 | – | – | – | |
| ACTH (pg/mL) (NR: 0–46) | 5 | – | – | – | |
| Cortisol (μg/dL) (NR: 5–25) | 7 | – | – | – | |
17α-OHP, 17-OH-progesterone; ACTH, adrenocorticotropic hormone; AMH, anti-Müllerian hormone; DHT, dihydrotestosterone; FSH, follicle stimulating hormone; HCG, human chorionic gonadotropin; LH, luteinizing hormone; NR, normal range; T, testosterone.
Twin A exhibited highly elevated gonadotrophins and low testosterone levels, even after human chorionic gonadotropin (HCG) stimulation test. Anti-müllerian hormone (AMH) and inhibin-B (Inh-B) concentrations were extremely low, consistent with gonadal dysgenesis. Baseline levels of adrenal androgens, 17-hydroxyprogesterone, adrenocorticotropic hormone (ACTH) and cortisol were within the normal range. Twin B had normal basal serum gonadotrophin and testosterone levels, which increased significantly after HCG stimulation. AMH and Inh-B levels were within the normal range for boys of the same age. Routine blood and urine testing indicated no abnormalities.
Imaging examinations
Ultrasound imaging revealed the absence of glands in Twin A’s labioscrotal folds, groin or pelvic cavity, but a prepubertal uterus (1.6 cm × 0.9 cm × 0.6 cm) was detected in the pelvis. Twin B had two testis-shaped masses located in the left groin (1.0 cm × 0.7 cm × 0.6 cm) and right scrotum (1.2 cm × 0.8 cm × 0.7 cm), along with a prepubertal uterus (2.7 cm × 1.2 cm × 0.7 cm). Head magnetic resonance imaging (MRI) scans of both twins showed slight enlargement of the bilateral lateral ventricles.
Surgical exploration
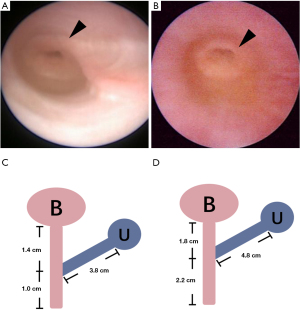
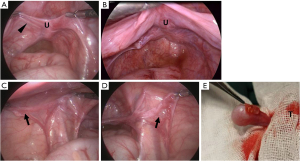
Urethroscopy revealed that the urethra and vagina converged into a single channel with a cervix visible at the top of the narrow vagina (Figure 2A,2B). The true urethras measured 1.4 cm for Twin A and 1.8 cm for Twin B (Figure 2C,2D). Laparoscopic exploration confirmed the presence of a uterus in both twins (Figure 3A,3B). Epididymis-like tissues, without gonads, were observed at bilateral internal rings of Twin A (Figure 3C,3D), which was consistent with the pathology after resection. Twin B’s gonads showed macroscopically masculine presentation (testes), devoid of epididymis and vas deferens. A necrotic vesicle on the right testicular head (Figure 3E) was resected and pathologically identified as papillary cystadenoma of the epididymis.
Genetic testing
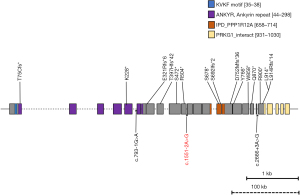
The karyotype of these identical twins was 46,XY. Whole-exome sequencing (WES) revealed a novel heterozygous de novo splice site variant in PPP1R12A gene, c.1551-2A>G (Figure 4). According to the American College of Medical Genetics and Genomics (ACMG) 2015 criteria (6), this variant is classified as “pathogenic”.
Gender assignment
The multidisciplinary team (MDT) engaged in shared decision-making with the parents regarding gender assignment for the affected twins. Following the evaluation of gonadal functionality and genitalia development, Twin A was assigned as female due to the absence of functional gonadal tissue and severely undervirilized external genitalia; while Twin B was assigned as male gender given the presence of well-functioning testes and relatively well-developed external genitalia.
Intervention and follow-up
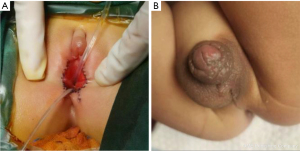
Twin A underwent vaginoplasty and urethroplasty to separate vagina and urethra at 30 months (Figure 5A). Meanwhile, Twin B underwent staged II hypospadias repair, left orchidopexy and right hernia repair (Figure 5B). To evaluate their psychosexual development, the Pre-School Activities Inventory (PSAI) scale (7) was applied to assess their gender role behaviors at 3.5 years old: Twin A scored 55.95 (neutral, slightly inclined to male), while Twin B scored 84.55 (male). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the parents of the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
UBMS is related to PPP1R12A and typically presents with brain and/or urogenital malformations. The UBMS individuals combined with genital abnormalities are associated with DSD, a group of rare conditions in which the chromosomal, gonadal, or phenotypical sex is incongruent (8). We herein present a case of 46,XY identical twins with DSD and UBMS caused by a novel heterozygous de novo splicing variant (c.1551-2A>G) in PPP1R12A. This variant results in the presence of a uterus in both 46,XY twins. Twin A exhibited gonadal dysgenesis and was assigned female, while Twin B had functional testes and was raised as male.
PPP1R12A encodes PPP1R12A (1030aa), also named myosin phosphatase-targeting subunit 1 (MYPT1), expressed extensively and strongly in the brain, thyroid, kidney, urinary tract, and other organ systems. It interacts with protein phosphatase type 1 catalytic unit (PPP1c) and M20/21 to form the heterotrimeric serine/threonine MP, the primary regulator of contractility in smooth muscle. Accumulating data support that PPP1R12A serves as the scaffold function of MP holoenzyme and a wide array of phosphatase substrates, and could possibly participate in the development, cell cycle, neurotransmitter release, and control of gene expression (1). Animal studies have shown that homologs of PPP1R12A are necessary for cell migration during dorsal closure and eye development in Drosophila (9), and their variety can result in embryonic death in mice (10). But these findings cannot be applied to human diseases directly. In situ hybridization and immunostaining demonstrated expression in the lower urinary tract and prosencephalic neural folds throughout crucial developmental stages in both human and murine fetuses (2).
To date, together with the twins reported here, a total of 20 individuals with 19 de novo loss-of-function variants in PPP1R12A have been reported (2-4). These variants were all pathogenic according to ACMG criteria, including 8 frameshift variants, 7 nonsense variants, 1 complex rearrangement, and 3 splice variants (Figure 4). These types of variants might activate the nonsense-mediated decay (NMD) mechanism, resulting in protein degradation, or they could lead to the premature termination of amino acid translation. According to the current data, due to the small number of cases coupled with the fact that the known variation sites are not concentrated in a specific structural region, there was no discernible relationship between genotype and phenotype. The phenotype of the same variant site is also different; for example, we reported that identical twin A has no testicles, while B has functional testicles.
The clinical characteristics of UBMS not only include brain abnormalities and urogenital malformations, but also developmental delay, intellectual disability, eye abnormalities, skeletal anomalies, and intestinal atresia, among others. Brain malformations in 7 individuals ranged from mild to severe, like agenesis of corpus callosum, leukomalacia, holoprosencephaly, and anencephaly. Developmental delay and head-facial features are more commonly observed, affecting 8 and 6 individuals, respectively. There are 4 individuals with brain abnormalities, developmental delays, and facial features. Four individuals were reported to have various eye abnormalities, the same number of reported cases as atresia of the digestive tract. There are only 4 individuals reported to have both brain abnormalities and urogenital malformations.
In 9 out of 14 individuals with 46,XY karyotype, Müllerian derivatives were not entirely regressed. The uterus is derived from Müllerian ducts and degenerates at 10 weeks of fetal development under the influence of AMH produced by Sertoli cells of the testis in XY individuals. The failure of Müllerian ducts degeneration can be attributed to mutations in the AMH or AMHR2 genes (11) or testis dysgenesis that causes the deficiency of AMH, such as the case in Twin A. In fact, PPP1R12A may be involved in Müllerian regression, because 5 patients, including Twin B, have well-developed testes and no AMH or AMHR2 gene variants, but have a uterus in the pelvic region. The known mechanism of Müllerian regression involves the migration of its epithelial cells to the surrounding mesenchyme when AMH acts on AMHR2 of the coelomic epithelium (12). This process of cell migration may require intact MP activity to enable cell motility, though this needs to be validated through experiments.
In the 14 46,XY individuals, 11 present under-masculinized external genitalia in varying degrees, and 6 out of the 11 individuals present testis dysgenesis, such as testicular hypertrophy or ovarian tissue. Normally, differentiation of fetal external genitalia occurs between 9 and 12 weeks of gestation in humans. The testosterone secreted by the testicles will cause the external genitalia of the fetus to develop in the male direction, and if there are no testicles, it will default to the female vulva. Twin A presented mild masculinization and testicular hypertrophy, indicating that in the early stages of external genital development, the testes were present but later atrophied. Twin B has the same genetic background, with better-functioning testes observed in vivo, as confirmed by an HCG stimulation test; however, the individual is not fully masculinized, indicating that testicular function is not sound during the critical period of differentiation. The reason why identical twins with the same genetic background have different gonad phenotypes (one testicle, one gonad atrophy) remains to be further elucidated.
Considering that the investigations are not recorded in the articles completely, such as karyotype, uterine exploration, and gonadal nature, the actual incidence is probably higher than it is now.
To prevent the anguish brought on by possible gender dysphoria, the greatest challenge in managing the twins was to assign them a gender that was appropriate and aligned with their future gender identity. Gender identity is the internal sense of self that a person experiences as male, female, or another gender (13). What exactly determines the gender: chromosomes, genes, gonads, sex phenotype, or anything else? According to previous research, the initial phase of sexual development begins with the establishment of the chromosomal sex at fertilization. Then, the sex-determining gene (SRY) on the distal part of the short arm of Y chromosome (Yp) directs the bipotential gonadal ridge into testes at embryo 5–7 weeks. The specific hormones (testosterone, AMH and Inh-B) secreted by the testicles masculinate the internal and external genitalia (sex phenotype) (14). Meanwhile, androgens play a direct role in organizing the brain during early development (12–24 weeks of fetal development; the first 3 months after birth), and pubertal androgens further stimulate and rearrange the brain’s preexisting structure, leading to masculine behaviors associated with male gender role (15). In the absence of the SRY gene on Yp, the embryonic gonad’s development will follow the female route, and the internal and external genitalia appear feminine spontaneously.
Psychosexual development is influenced by various factors, such as brain anatomy, hormonal and genetic and influences, and social and familiar circumstances (8). During this complex and long-lasting process, functional testicular tissue plays a major part in male gender identity and male gender role behavior. It is relatively reliable to give a gender assignment according to the function of the gonads.
Rationally, the timing of gender assignment should be postponed until the child reaches adulthood and can determine his or her own gender (16). In fact, there is no recognized third gender in China other than male or female. Undiagnosed and untreated DSD children often suffer severe social discrimination and may develop gender dysphoria. It is recommended to assign the gender during early childhood. For those with a clear genetic diagnosis, there are recommendations for gender assignment in the guidelines (15,17).
An open discussion regarding gender assignment took place between the MDT and the parents. Specialists from the departments of endocrinology, urology, gynecology, psychology, and genetics participated in the MDT consultation, which focused on diagnostic evaluation and surgical planning. Twin A was assigned to be a girl due to gonadal atrophy, underwent female genitoplasty and required hormonal replacement at the onset of puberty and beyond. Twin B continued to be reared as a boy because his testicles were functioning well, accepted hypospadias repair surgery.
Following up with these twins is crucial, especially monitoring their psychosexual development. PSAI is designed to evaluate their gender role behavior of 2–7 years old children. By the time they were 3.5 years old, Twin A’s gender role behavior was neutral and Twin B’s was male. Close follow-up of the twins continues at our clinic.
Conclusions
We report the first case of monozygotic twins with a novel de novo PPP1R12A variant (c.1551-2A>G), leading to UBMS and DSD. Despite the same gene background, the twins exhibited distinct genital phenotypes and were assigned different genders. Our findings offer critical insights into gender assignment and management strategies for UBMS-associated DSD cases.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-166/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-166/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-166/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s), and with the Helsinki Declaration and its subsequent amendments. Written informed consent was obtained from the parents of the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kiss A, Erdődi F, Lontay B. Myosin phosphatase: Unexpected functions of a long-known enzyme. Biochim Biophys Acta Mol Cell Res 2019;1866:2-15. [Crossref] [PubMed]
- Hughes JJ, Alkhunaizi E, Kruszka P, et al. Loss-of-Function Variants in PPP1R12A: From Isolated Sex Reversal to Holoprosencephaly Spectrum and Urogenital Malformations. Am J Hum Genet 2020;106:121-8. [Crossref] [PubMed]
- Diao Y, Sun W, Zhang Z, et al. Clinical report and genetic analysis of a neonate with genitourinary and/or brain malformation syndrome caused by a non-coding sequence variant of PPP1R12A. Mol Genet Genomic Med 2023;11:e2223. [Crossref] [PubMed]
- Picard JY, Morin G, Devouassoux-Shisheboran M, et al. Persistent Müllerian duct syndrome associated with genetic defects in the regulatory subunit of myosin phosphatase. Hum Reprod 2022;37:2952-9. [Crossref] [PubMed]
- Ahmed SF, Khwaja O, Hughes IA. The role of a clinical score in the assessment of ambiguous genitalia. BJU Int 2000;85:120-4. [Crossref] [PubMed]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- Golombok S, Rust J. The measurement of gender role behaviour in pre-school children: a research note. J Child Psychol Psychiatry 1993;34:805-11. [Crossref] [PubMed]
- Lee PA, Houk CP, Ahmed SF, et al. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics 2006;118:e488-500. [Crossref] [PubMed]
- Tan C, Stronach B, Perrimon N. Roles of myosin phosphatase during Drosophila development. Development 2003;130:671-81. [Crossref] [PubMed]
- Okamoto R, Ito M, Suzuki N, et al. The targeted disruption of the MYPT1 gene results in embryonic lethality. Transgenic Res 2005;14:337-40. [Crossref] [PubMed]
- Picard JY, Cate RL, Racine C, et al. The Persistent Müllerian Duct Syndrome: An Update Based Upon a Personal Experience of 157 Cases. Sex Dev 2017;11:109-25. [Crossref] [PubMed]
- Zhan Y, Fujino A, MacLaughlin DT, et al. Müllerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Müllerian duct regression. Development 2006;133:2359-69. [Crossref] [PubMed]
- Zucker KJ. Intersexuality and gender identity differentiation. J Pediatr Adolesc Gynecol 2002;15:3-13. [Crossref] [PubMed]
- Kim SS, Kolon TF. Hormonal abnormalities leading to disorders of sexual development. Expert Rev Endocrinol Metab 2009;4:161-72. [Crossref] [PubMed]
- Meyer-Bahlburg HF, Baratz Dalke K, Berenbaum SA, et al. Gender Assignment, Reassignment and Outcome in Disorders of Sex Development: Update of the 2005 Consensus Conference. Horm Res Paediatr 2016;85:112-8. [Crossref] [PubMed]
- Khorashad BS, Gardner M, Lee PA, et al. Recommendations for 46,XY Disorders/Differences of Sex Development Across Two Decades: Insights from North American Pediatric Endocrinologists and Urologists. Arch Sex Behav 2024;53:2939-56. [Crossref] [PubMed]
- Lee PA, Nordenström A, Houk CP, et al. Global Disorders of Sex Development Update since 2006: Perceptions, Approach and Care. Horm Res Paediatr 2016;85:158-80. [Crossref] [PubMed]


