
Functional cure in a child with chronic hepatitis B with rtM204I mutation through an atypical serological response: a case report
Highlight box
Key findings
• Long-acting interferon plus tenofovir disoproxil fumarate (TDF) achieved functional cure in a child with rtM204I mutation and atypical serology.
What is known and what is new?
• Lamivudine resistance mutations (e.g., rtM204I) limit treatment efficacy and are common with prolonged nucleos(t)ide analog (NA) therapy.
• Combination therapy can overcome resistance and induce hepatitis B surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) clearance in children.
What is the implication, and what should change now?
• Early, tailored treatment in children with atypical serological patterns enhances prognosis and supports guideline updates.
Introduction
Background
A functional cure for chronic hepatitis B (CHB) is the desired goal of the currently available antiviral therapies. It is defined as persistent hepatitis B surface antigen (HBsAg) negativity, hepatitis B virus (HBV) DNA levels below the lower detection limit, and the possible presence of covalently closed circular DNA (cccDNA) within hepatocyte nuclei. Children often have higher cure rates after antiviral therapy because of immunological and virological factors (1). However, rtM204I mutation—a well-known drug resistance site in HBV reverse transcriptase region—can cause virological breakthroughs that limit treatment options.
Rationale and knowledge gap
Although adult studies indicate that pegylated interferon α (PegIFNα)-based therapy may be an effective salvage treatment (2,3), data on this approach in children are extremely limited. This highlights the need for further assessment of treatment outcomes in pediatric patients with drug-resistant CHB.
Objective
This report describes the clinical and serological course of a pediatric patient with CHB harboring the rtM204I viral mutation. The case is characterized by atypical serological patterns, including double positivity for HBsAg and antibody to hepatitis B surface antigen (anti-HBs) as well as response dissociation with HBsAg disappearing before hepatitis B e antigen (HBeAg) when adjusting the tenofovir disoproxil fumarate (TDF) and PegIFNα-2a combination therapy—demonstrating a sustained functional cure. The results of this case report offer novel insights into future individualized treatment strategies for drug-resistant CHB in children. We present this case in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-250/rc).
Case presentation
Patient data
A 4-year-old boy presented for regular follow-up and treatment after testing positive for HBsAg 3 years ago. His mother was found to have CHB, and his father was healthy. The infection was acquired through vertical transmission (mother-to-child). Despite receiving a complete hepatitis B vaccination series at 0, 1, and 6 months, this case represented a breakthrough infection. The patient had no history of other chronic diseases.
Diagnosis and treatment
HBsAg positivity was initially detected during a health check-up at 1 year and 7 months of age, and further examination confirmed CHB. The laboratory test results were as follows: HBsAg, 14,446.85 IU/mL (reference value <0.05 IU/mL); HBeAg, 2,485.63 Paul Ehrlich Institute Units (PEIU)/mL (reference value <0.59 PEIU/mL); HBV DNA, 8.99×109 IU/mL (reference value <20 IU/mL); alanine aminotransferase (ALT) level, 77 U/L (mildly elevated), and genotype: type C.
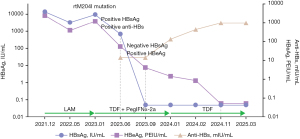
Owing to the young age of the patient, lamivudine (LAM) monotherapy was initiated. After 6 months of LAM treatment, the levels of HBV DNA, HBsAg, and HBeAg declined to 2.85×104 IU/mL, 3,400.36 IU/mL, and 404.39 PEIU/mL, respectively. However, after 12 months, the levels of HBV DNA, HBsAg, and HBeAg increased to 4.66×107 IU/mL, 9,627.31 IU/mL, and 1,200.69 PEIU/mL, respectively. Sanger sequencing detected M204I mutation in the HBV polymerase region, indicating LAM resistance.
Because of the M204I mutation, the treatment was adjusted to TDF combined with PegIFNα-2a at 90 µg. After 12 weeks of combination therapy, the levels of HBV DNA, HBsAg, and HBeAg decreased to 1.75×102 IU/mL, 702.23 IU/mL, and 54.79 PEIU/mL, respectively, and anti-HBs antibodies became positive (14.51 mIU/mL). After 24 weeks of combination therapy, the levels of HBV DNA and HBeAg declined to <20 IU/mL (below the detection limit) and 4.22 PEIU/mL, respectively. HBsAg became negative, whereas anti-HBs remained positive (14.28 mIU/mL). After 36 weeks of combination therapy, HBV DNA remained undetectable, HBsAg was negative, anti-HBs was positive (the titer increased to 137.85 mIU/mL), and HBeAg levels declined to 1.45 PEIU/mL.
The treatment was readjusted, with PegIFNα-2a being discontinued while TDF monotherapy continued. After 2 months of TDF treatment, HBV DNA remained undetectable, HBsAg was negative, anti-HBs was positive (the titer increased to 436.75 mIU/mL), and HBeAg levels declined to 0.85 PEIU/mL. After 10 months of TDF treatment, HBV DNA remained undetectable, HBsAg remained negative, anti-HBs was positive (the titer increased to >1,000 mIU/mL), HBeAg became negative without seroconversion, and ALT levels normalized, thus achieving a functional cure.
Follow-up and monitoring
Follow-up was conducted 3 months after achieving a functional cure. The results demonstrate that HBV DNA remained below the detection limit (<20 IU/mL), HBsAg was negative, and anti-HBs was positive (titer >1,000 mIU/mL). HBeAg was negative, anti-HBe was positive, ALT and aspartate aminotransferase (AST) levels remained normal, and liver ultrasound revealed no significant abnormalities. The dynamics of serological markers during treatment and follow-up are presented in Figure 1.
This study was approved by the Ethics Committee of The Second Hospital & Clinical Medical School, Lanzhou University (No. 2025A-389). All procedures performed in this study were in accordance with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the parents of the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Significance of rtM204I mutation
The tyrosine-methionine-aspartate-aspartate (YMDD) motif located in the core active region of HBV reverse transcriptase is crucial for HBV DNA replication. During prolonged treatment with LAM or other nucleos(t)ide analogs (NAs), HBV can undergo M204I mutation, resulting in substitution of the methionine (M) with isoleucine (I) in the YMDD sequence to form a tyrosine-isoleucine-aspartate-aspartate sequence. This substitution confers LAM resistance to the virus. The rate of LAM resistance increases with the duration of treatment, reaching 38%, 49%, and 65% after 2, 3, and 5 years, respectively (4).
Unlike NAs, which directly inhibit the reverse transcription of viral DNA, PegIFNα can activate the host’s anti-HBV cellular immune response to kill infected liver cells and accelerate cccDNA clearance through non-cytopathic effects (5). To assess LAM resistance, a previous study compared the efficacy of long-acting interferon in treatment-naive patients who are HBeAg-positive and those with LAM resistance, finding no difference in HBeAg seroconversion rates and HBV DNA levels <100,000 copies/mL between the two groups (2). Therefore, adding PegIFNα-2a to NA therapy may be an appropriate salvage treatment option for patients with NA resistance (3). In this case, after rtM204I mutation was detected, the treatment was promptly adjusted to TDF combined with PegIFNα-2a. After 24 weeks of this combination therapy, the HBV DNA levels were below the detection limit, HBsAg became negative, and the HBeAg levels decreased significantly and eventually became negative, resulting in a favorable therapeutic outcome.
Relationship between HBsAg positivity and anti-HBs positivity, and HBsAg negativity and HBeAg positivity
The presence of HBsAg in the blood indicates HBV infection, whereas the appearance of neutralizing antibody (anti-HBs) indicates infection clearance. However, in this patient, anti-HBs positivity was detected as early as the 12th week of combination therapy with PegIFNα-2a, whereas the seroconversion to HBsAg negativity occurred later, specifically in the 24th week. Large cohort studies have reported the coexistence of HBsAg and anti-HBs in 2.8–5.8% of cases (6-8), and this may be associated with HBV genotype C-specific epitope mutations, “a” determinant mutations, HBsAg isoforms, and viral immune escape. The simultaneous presence of both does not necessarily indicate complete HBV infection clearance. Therefore, subsequent monitoring is clinically significant (9). For such patients, the guidelines recommend monitoring and treatment as if anti-HBs is negative (10,11). Notably, anti-HBs is an independent predictor of HBsAg disappearance and HBeAg clearance, and anti-HBs-positive children with CHB are more likely to have a favorable prognosis (12). In this case, the child exhibited dual positivity for HBsAg and anti-HBs at the 12th week of TDF combined with PegIFNα-2a therapy. Continued combination therapy resulted in HBsAg disappearance and eventual HBeAg clearance. The treatment outcome indicates that children with this serological pattern have a higher likelihood of achieving a functional cure and should actively receive treatment.
Unlike HBsAg, which has two sources—cccDNA and integrated HBV DNA—HBeAg production depends on active transcription of cccDNA. Typically, HBsAg disappearance occurs after HBeAg clearance and sustained HBV DNA undetectability. However, in the reported patient, seroconversion to HBsAg negativity occurred in the 24th week of combination therapy, but HBeAg remained positive until the 36th week of PegIFNα-2a combination therapy and only became negative after 10 months of TDF monotherapy consolidation treatment. Additionally, among patients who achieve HBsAg clearance during interferon therapy, 6.5–20% can still test positive for HBeAg, indicating incomplete HBV immune control and a need for continued antiviral and consolidation therapy (13,14). The reasons why HBeAg remains positive after HBsAg disappears may include the following: (I) rtA181T and rtM204I mutations cause substitutions in the S domain, specifically sW172* and sW196* that act as stop codons, reducing HBsAg secretion. HBsAg levels can be affected by drug-resistant mutations in the reverse transcriptase region (15). (II) Chronic infection with genotype C HBV requires extensive hepatocyte lysis to achieve HBeAg seroconversion. Compared with genotype B infection, patients with genotype C HBV infection have a lower and later rate of HBeAg seroconversion (16). (III) Under PegIFN treatment and low viral load conditions, HBV can repress transcription from the surface promoter SP1/SP2—resulting in HBsAg disappearance—through mutations and/or epigenetic alterations. Simultaneously, it may enhance the transcriptional activity at the basal core promoter region—resulting in persistent HBeAg positivity and facilitating cccDNA replenishment. This helps maintain chronic HBV infection (14), offering greater insights into the atypical serological response pattern—HBsAg disappeared before HBeAg, and we termed this phenomenon “HBsAg and HBeAg response dissociation”.
The Chinese guidelines for the prevention and treatment of CHB define a functional cure as persistent HBsAg negativity after treatment cessation, regardless of the appearance of anti-HBs, HBV DNA levels below the lower detection limit, normal levels of liver biochemical indicators, and the possible presence of cccDNA within hepatocyte nuclei (17). Additionally, western guidelines emphasize HBsAg and HBV DNA levels being below the detection limit (10,11). The 2022 AASLD-EASL Hepatitis B and C Treatment Endpoints Conference further specifies that HBsAg and HBV DNA should remain persistently undetectable 24 weeks after treatment cessation, without strict regulations regarding the HBeAg status (18). However, the HBeAg status at the end of long-acting interferon therapy is indicated as an independent predictor of HBsAg reactivation (13,19). Additionally, the renowned American hepatologist Professor Anna Suk-Fun Lok has stated that HBeAg clearance should be a criterion for functional cure (20). Therefore, patients who remain HBeAg-positive despite HBsAg clearance during antiviral treatment should not be considered functionally cured. Treatment should continue until HBeAg clearance, regardless of seroconversion.
Characteristics of CHB treatment in children
Approximately 95% of infants infected with HBV at birth or before the age of 1 year develop CHB. China has historically had a high prevalence of HBV infection, primarily because of vertical transmission (mother-to-child) and infections acquired during infancy. These patients often do not receive antiviral treatment until adulthood because they may not exhibit significant symptoms earlier in life. Although current antiviral treatments can effectively suppress viral replication in adult patients, achieving a functional cure remains challenging. PegIFNα-2a treatment is effective and well tolerated in children with CHB during the immune-active phase, achieving a higher rate of HBsAg clearance than that in adults (21,22). Additionally, a significant proportion of children in the immune-tolerant phase or those with normal or mildly elevated ALT levels achieve undetectable serum HBV DNA levels, HBeAg seroconversion, and HBsAg disappearance when treated with PegIFNα (23,24). Moreover, the age at which treatment is initiated is negatively associated with HBsAg disappearance, with children aged 1–6 years exhibiting higher cure rates than those aged 7–14 years (22). In infants with chronic HBV infection and elevated transaminase levels, antiviral treatment before the age of 1 year can facilitate rapid HBsAg clearance (25). CHB progresses because of interaction between the virus and the host immune system. Persistent liver inflammation causes repeated cycles of hepatocyte death and regeneration, resulting in hepatocytes with integrated HBV DNA that have a proliferative advantage and form clonal colonies. These hepatocytes can express HBsAg, making CHB challenging to cure in adults. In contrast, children have less integrated HBV DNA in their liver tissues and have not experienced the prolonged immune cell exhaustion caused by viral antigen stimulation, as occurs in adults. Additionally, early antiviral treatment prevents HBV infection in novel hepatocytes, which may explain the higher CHB cure rates in children (1). Unlike current international guidelines that recommend antiviral treatment only for children with HBeAg-positive CHB (immune clearance phase) and not for those in the immune-tolerant phase (10,17,26,27), the updated Chinese guidelines have broadened treatment eligibility, specifically for children aged 1–7 years (17). Large prospective cohort studies are required to provide a theoretical basis for CHB treatment in children.
The limitation of this case is that liver biopsy was not performed on the child, hindering our ability to understand the extent of liver tissue inflammation and fibrosis, as well as the levels of intrahepatic cccDNA, HBsAg, HBV DNA integration, and transcription. Therefore, we were unable to predict the risk of recurrence and determine whether complete cure was achieved.
Conclusions
We report a case of a child with rtM204I mutation and CHB who exhibited atypical serological patterns during treatment with TDF and long-acting interferon. These patterns included double positivity for HBsAg and anti-HBs and response dissociation, whereby HBsAg disappeared before HBeAg. Therefore, the child achieved HBsAg and HBeAg seroconversion, HBV DNA levels <20 IU/mL, and normalized ALT levels, resulting in a sustained functional cure. This indicates that long-acting interferon has significant therapeutic value in children with drug-resistant mutations. Further studies are needed, however, to confirm and reveal the underlying mechanism. For the time being, making appropriate clinical decisions in response to atypical serological patterns during treatment can help achieve a functional cure and enhance the long-term prognosis.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-250/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-250/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-250/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of The Second Hospital & Clinical Medical School, Lanzhou University (No. 2025A-389). All procedures performed in this study were in accordance with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the parents of the patient for publication of this case report. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang FS, Li J, Zhang C. Why is the functional cure rate of young children with chronic hepatitis B receiving antiviral therapy considerably high? Hepatol Int 2024;18:296-8. [Crossref] [PubMed]
- Suh DJ, Lee HC, Byun KS, et al. Efficacy and safety of pegylated interferon-α2a in patients with lamivudine-resistant HBeAg-positive chronic hepatitis B. Antivir Ther 2013;18:765-73. [Crossref] [PubMed]
- Liu Y, Li W, Jia T, et al. Sustained Responses in Chronic Hepatitis B Patients with Nucleos(t)ide Analogue Drug-resistance after Peg-interferon Alfa-2a Add-on Treatment: A Long-term Cohort Study. J Clin Transl Hepatol 2018;6:18-24. [Crossref] [PubMed]
- Kasırga E. Lamivudine resistance in children with chronic hepatitis B. World J Hepatol 2015;7:896-902. [Crossref] [PubMed]
- Hu JL, Huang AL. Classifying hepatitis B therapies with insights from covalently closed circular DNA dynamics. Virol Sin 2024;39:9-23. [Crossref] [PubMed]
- Hou W, Huo Z, Du Y, et al. Characteristics of amino acid substitutions within the "a" determinant region of hepatitis B virus in chronically infected patients with co-existing HBsAg and anti-HBs. Clinics and Research in Hepatology and Gastroenterology 2020;44:923-31. [Crossref] [PubMed]
- Colson P, Borentain P, Motte A, et al. Clinical and virological significance of the co-existence of HBsAg and anti-HBs antibodies in hepatitis B chronic carriers. Virology 2007;367:30-40. [Crossref] [PubMed]
- Pu Z, Li D, Wang A, et al. Epidemiological characteristics of the carriers with coexistence of HBsAg and anti-HBs based on a community cohort study. J Viral Hepat 2016;23:286-93. [Crossref] [PubMed]
- Zhu D, Chen W, Xu C, et al. Virology and Serological Characteristics of Chronic Hepatitis B Patients with the Co-existence of HBsAg and Anti-HBs Antibodies. Clin Lab 2020; [Crossref]
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-99. [Crossref] [PubMed]
- EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98. [Crossref] [PubMed]
- Gu Y, Li S, Yao Z, et al. Characteristics and clinical treatment outcomes of chronic hepatitis B children with coexistence of hepatitis B surface antigen (HBsAg) and antibodies to HBsAg. BMC Med 2024;22:77. [Crossref] [PubMed]
- Li M, Sun F, Bi X, et al. Consolidation treatment needed for sustained HBsAg-negative response induced by interferon-alpha in HBeAg positive chronic hepatitis B patients. Virol Sin 2022;37:390-7. [Crossref] [PubMed]
- Jiang B, Guan G, Zhao K, et al. Mechanisms underlying delayed loss of HBeAg and HBV DNA following HBsAg seroclearance in PEG-IFNα treated patients of chronic hepatitis B. Emerg Microbes Infect 2025;14:2475847. [Crossref] [PubMed]
- Shen H, Chen C, Ye C, et al. Mutations in reverse transcriptase region of HBV affect Hepatitis B surface antigen titers and its correlation with HBV DNA. J Infect Dev Ctries 2019;13:1062-7. [Crossref] [PubMed]
- Chu CM, Liaw YF. Chronic hepatitis B virus infection acquired in childhood: special emphasis on prognostic and therapeutic implication of delayed HBeAg seroconversion. J Viral Hepat 2007;14:147-52. [Crossref] [PubMed]
- Hong Y, Fusheng W, Taisheng L. Guidelines for the prevention and treatment of chronic hepatitis B. Chinese Journal of Hepatology 2022;30:1309-31. [Crossref] [PubMed]
- Ghany MG, Buti M, Lampertico P, et al. Guidance on treatment endpoints and study design for clinical trials aiming to achieve cure in chronic hepatitis B and D: Report from the 2022 AASLD-EASL HBV-HDV Treatment Endpoints Conference. Hepatology 2023;78:1654-73. [Crossref] [PubMed]
- Guo Y, Han J, Zhang Y, et al. End-of-treatment anti-HBs levels and HBeAg status identify durability of HBsAg loss after PEG-IFN discontinuation. Front Cell Infect Microbiol 2023;13:1120300. [Crossref] [PubMed]
- Lok ASF. Toward a Functional Cure for Hepatitis B. Gut Liver 2024;18:593-601. [Crossref] [PubMed]
- Wirth S, Zhang H, Hardikar W, et al. Efficacy and Safety of Peginterferon Alfa-2a (40KD) in Children With Chronic Hepatitis B: The PEG-B-ACTIVE Study. Hepatology 2018;68:1681-94. [Crossref] [PubMed]
- Wang L, Zhao J, Liu J, et al. Long-term benefits of interferon-α therapy in children with HBeAg-positive immune-active chronic hepatitis B. J Viral Hepat 2021;28:1554-62. [Crossref] [PubMed]
- Zhu S, Zhang H, Dong Y, et al. Antiviral therapy in hepatitis B virus-infected children with immune-tolerant characteristics: A pilot open-label randomized study. J Hepatol 2018;68:1123-8. [Crossref] [PubMed]
- Li J, Fan P, Xu Z, et al. Functional Cure of Chronic Hepatitis B with Antiviral Treatment in Children having High-level Viremia and Normal or Mildly Elevated Serum Aminotransferase. J Clin Transl Hepatol 2023;11:1011-22. [Crossref] [PubMed]
- Liu LZ, Sun J. Early initiation of antiviral therapy contributes to a rapid and significant loss of serum HBsAg in infantile-onset hepatitis B. J Hepatol 2019;71:1263-4. [Crossref] [PubMed]
- Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98. [Crossref] [PubMed]
- Sokal EM, Paganelli M, Wirth S, et al. Management of chronic hepatitis B in childhood: ESPGHAN clinical practice guidelines: consensus of an expert panel on behalf of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Hepatol 2013;59:814-29. [Crossref] [PubMed]


