
Analysis of factors influencing mortality in immunocompetent children with severe adenovirus pneumonia undergoing conventional treatments: a retrospective cohort study
Highlight box
Key findings
• Among immunocompetent children with severe adenovirus pneumonia (SAP) receiving conventional treatments, decreased oxygen saturation (SpO2) elevated partial pressure of carbon dioxide in arterial blood (PaCO2), and pulmonary consolidation affecting two or more lobes were identified as independent risk factors for mortality.
• The mortality rate in children whose conventional treatments failed reached 43.75%, and all of them did not receive extracorporeal membrane oxygenation (ECMO).
What is known and what is new?
• SAP can cause life-threatening complications in immunocompetent children, and some patients deteriorate despite conventional treatments such as immunomodulatory therapy and mechanical ventilation.
• This study is among the first to isolate immunocompetent children treated solely with conventional therapies, identifying specific predictors of death. The findings suggest that certain clinical and radiological features can help identify children at high risk of treatment failure early.
What is the implication, and what should change now?
• Clinicians should closely monitor SpO2, PaCO2, and extent of lung consolidation in SAP cases. The presence of these high-risk factors may indicate that conventional treatment alone is insufficient.
• In such scenarios, early consideration of advanced supportive therapies, such as ECMO, may improve survival outcomes. These results support a more proactive, individualized treatment strategy for SAP in resource-limited settings.
Introduction
Adenovirus (AdV) is one of the most common viruses responsible for respiratory infections, accounting for 4–10% of pediatric pneumonia cases (1,2). While AdV infections are usually self-limiting in most immunocompetent patients, respiratory failure develops in 10–30% of severe AdV pneumonia (SAP) cases, with fatality rates exceeding 50% (3,4). In the acute stage, respiratory failure, extensive pulmonary consolidation, often accompanied by multiple organ dysfunction syndrome (MODS), significantly contributes to mortality in young children with SAP (5-7).
In clinical practice, the current conventional treatments for AdV pneumonia include antiviral drugs, immunomodulatory therapy, bronchoscopy, respiratory support, and blood purification (7,8). However, the use of antiviral drugs remains a topic of debate (9). Children with SAP are typically administered immunomodulatory therapies such as glucocorticoids and intravenous immunoglobulin, alongside broad-spectrum antibiotics in cases of bacterial coinfection (7). For SAP patients who develop dyspnea due to pulmonary consolidation or atelectasis, bronchoscopy and/or mechanical ventilation (MV) support is recommended (3). Additionally, blood purification may be considered for SAP patients with an overactive immune response (7). Despite positive conventional treatments, some children with SAP continue to experience clinical deterioration (3,10). In recent years, more and more children with SAP have received the new method of extracorporeal membrane oxygenation (ECMO); however, its efficiency remains controversial. Some studies (11,12) report high mortality rates (58–62%) in pediatric SAP patients who require ECMO, while other studies suggest that ECMO may offer effective support, with reduced mortality rates (25.00–33.33%) (3,13).
In Guangzhou Women and Children’s Medical Centre, serial clinical studies of SAP have been done previously with analyses from different aspects in order to understand the different characteristics of SAP in comprehensive ways (2,14-16). Most previous studies do not isolate immunocompetent children or those receiving only conventional therapies. In the present study, we analyzed 156 SAP pediatric patients, including 14 children who did not receive ECMO therapy because of their rapid deterioration and died despite all the conventional treatments administered. This study aimed to assess the factors influencing mortality in immunocompetent children with SAP undergoing conventional therapies and to fill that gap and help identify high-risk children who may not benefit from conventional treatments, emphasizing its potential contribution to clinical decision-making. Our findings may be beneficial for the development of early management strategies and help optimize the utilization of limited healthcare resources, particularly in developing countries. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-184/rc).
Methods
Objectives and data collection
This study included 156 pediatric SAP patients who were admitted to Guangzhou Women and Children’s Medical Centre between January 2019 and December 2020. Of these patients, 14 underwent conventional treatments that failed and who subsequently died (referred to as the “conventional treatments death group”). This group was compared with a control group of 142 SAP patients who were successfully treated with conventional therapies (referred to as the “conventional treatments survivor group”). Children with human immunodeficiency virus (HIV) infections, inborn errors of immunity, hematologic malignancies, and comorbidities such as neuromuscular diseases with immunocompromisation, autoimmune diseases, and inherited metabolic diseases were excluded from the study. We excluded cases with incomplete datasets, such as children who left against medical advice or died within 24 h of admission and any patient managed with ECMO.
Demographic, clinical, laboratory, radiological, microbiological, complication, treatment, and outcome data were retrospectively retrieved and analyzed for all patients and individual consent for this retrospective analysis was waived. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of the Guangzhou Women and Children’s Medical Center at Guangzhou Medical University (2024NO.404A01).
Diagnostic methods and setting
Patients were identified based on positive AdV detection in nasopharyngeal secretions using polymerase chain reaction. Serum-specific antibody tests confirmed HIV-negative status in all cases. Indirect immunofluorescence was employed during the acute illness to identify other viral respiratory pathogens in nasopharyngeal secretions. Sputum and/or bronchoalveolar lavage fluid (BALF) samples were cultured to identify potential bacterial and Mycoplasma pneumoniae infections. All patients received chest X-rays, while high-resolution computed tomography (CT) was performed in selected cases with extensive radiographic findings. Additionally, bronchoscopy was performed in patients who exhibited widespread pulmonary consolidation or atelectasis on high-resolution computed tomography (HRCT).
The diagnosis of severe pneumonia followed the British Thoracic Society guideline criteria (17). The definition of conventional treatments followed the Practice Guideline for Adenovirus Pneumonia in Children [2019] in China, which includes antiviral drugs, immunomodulatory therapy, bronchoscopy, respiratory support, and blood purification (8), because the use of antiviral drugs remains a topic of debate, all the patients in our study did not receive the antiviral drugs. The use of ECMO was defined as advanced life support treatment beyond conventional treatments and was guided by the recommendations of the Extracorporeal Life Support Organization (ELSO) (18). The definition of acute respiratory distress syndrome (ARDS) followed the guidelines proposed by the Pediatric Acute Lung Injury Consensus Conference (11), while septic shock was defined according to the International Pediatric Sepsis Consensus Conference guidelines (19).
Statistical analyses
The cohort consisted of 156 pediatric patients diagnosed with SAP between 2019 and 2020 in Guangzhou Women and Children’s Medical Center of Guangzhou Medical University. The normality of continuous variables was evaluated using the Kolmogorov-Smirnov test. Normally distributed continuous variables were expressed as mean ± standard deviation (SD), while categorical variables were presented as frequencies and percentages. To delineate the characteristics of survivors and non-survivors, the following statistical methods were used: for normally distributed continuous variables with homogeneous variances, the Student’s t-test was utilized. For variables with heterogeneous variances, an adjusted t-test was applied. Non-normally distributed or heterogeneous continuous variables were analyzed using the Wilcoxon rank-sum test. Categorical variables were analyzed using the chi-squared test for 2×2 contingency tables or an R-by-C chi-squared test, depending on the dimensions of the table.
To assess the independent predictive power of each variable, multivariable logistic regression models were developed. A binary outcome variable was defined, categorizing patients as survivors or non-survivors. Variables were included in the model based on an inclusion criterion of α1=0.05 and excluded based on α2=0.10, depending on their statistical significance. All statistical analyses were conducted using SPSS version 20.0, with the level of statistical significance set at α=0.05 for all tests and all P values are two-sided.
Results
Demographics and clinical characteristics
Figure 1 shows the flowchart of patient enrollment. Over the two-year period, hospitalized children tested positive for AdV in nasopharyngeal secretions. A total of 30 patients were excluded according to the predefined criteria. The demographics and clinical characteristics of the 156 hospitalized SAP patients, including 142 patients in the conventional treatment survivor group and 14 patients in the conventional treatment death group, are presented in Table 1. The study cohort included 96 males (61.54%), with a mean age of 30.12±28.78 months (range, 1–144 months). Fever and cough were present in all patients (100%, 156/156), with shortness of breath noted in 36.54% (57/156), vomiting or diarrhea in 20.51% (32/156), and cyanosis in 15.38% (24/156). The mean duration of fever was 14.32±7.52 days. The mean oxygen saturation (SpO2) was 89.60±10.74 %. Crackles were the most common physical sign, observed in 87.18% (136/156) of patients, followed by tachycardia (heart rate >180/min for ≤1 year or >160/min for >1 year) in 39.10% (61/156), tachypnea (respiratory rate >70/min for ≤1 year or >60/min for >1 year) in 36.54% (57/156), and wheezing in 19.87% (31/156).
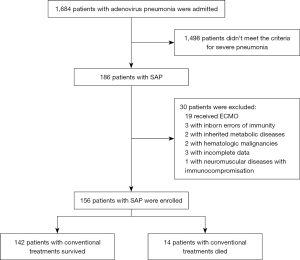
Table 1
| Characteristics | Conventional treatments | P value | ||
|---|---|---|---|---|
| Total (N=156) | Survivor group (N=142) | Death group (N=14) | ||
| Demographics | ||||
| Male gender | 96 (61.54) | 84 (59.15) | 12 (85.71) | 0.06 |
| Age (months) | 30.12±28.78 | 31.94±28.85 | 19.00±12.51 | 0.12 |
| Clinical characteristics | ||||
| Clinical symptoms | ||||
| Fever | 156 (100.00) | 142 (100.00) | 14 (100.00) | >0.99 |
| Fever (days) | 14.32±7.52 | 13.89±6.47 | 15.71±10.63 | 0.93 |
| Cough | 156 (100.00) | 142 (100.00) | 14 (100.00) | >0.99 |
| Shortness of breath | 57 (36.54) | 43 (30.28) | 14 (100.00) | <0.001* |
| Vomiting/diarrhea | 32 (20.51) | 27 (19.01) | 5 (35.71) | 0.26 |
| Cyanosis | 24 (15.38) | 10 (7.04) | 14 (100.00) | <0.001* |
| Seizures | 4 (2.56) | 3 (2.11) | 1 (7.14) | 0.80 |
| Physical exam findings | ||||
| Respiratory rate >70/min (≤1 year) or >60/min (>1 year) | 57 (36.54) | 43 (30.28) | 14 (100.00) | <0.001* |
| Heart rate >180/min (≤1 year) or >160/min (>1 year) | 61 (39.10) | 51 (35.92) | 10 (71.43) | 0.01* |
| Oxygen saturation (%) | 89.60±10.74 | 93.09±8.17 | 72.43±25.17 | <0.001* |
| Decreased blood pressure | 11 (7.05) | 3 (2.11) | 8 (57.14) | <0.001* |
| CRT >3 s | 12 (7.69) | 6 (4.23) | 6 (42.85) | <0.001* |
| Crackles | 136 (87.18) | 124 (87.32) | 12 (85.71) | >0.99 |
| Wheezing | 31 (19.87) | 27 (19.01) | 4 (28.57) | 0.61 |
Categorical variables are presented as n (%) and continuous variables are presented as mean ± standard deviation. *, statistically significant. CRT, capillary refill time; SAP, severe adenovirus pneumonia.
Laboratory, radiological, and microbiological findings
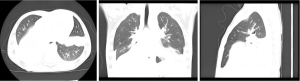
Table 2 presents abnormal laboratory, radiological, and microbiological findings. The mean partial pressure of oxygen (PO2) and the mean partial pressure of carbon dioxide (PCO2) in the cohort of 156 patients were 9.35±2.12 and 5.13±2.08 kPa, respectively. Mean values for white blood cell (WBC) count and hemoglobin were (8.05±5.11)×109/L and 104.32±15.58 g/L, respectively. Diffuse infiltrative changes were observed in both lungs on chest scans, with consolidation observed in 80.76% (126/156) of patients. In the conventional treatment death group, 92.86% (13/14) exhibited consolidation in two or more lobes (Figure 2) (P<0.05). Additional radiographic findings included pleural effusion (48.72%, 76/156) and pneumothorax (5.77%, 9/156). Besides Adv, 33.97% (53/156) had M. pneumoniae coinfection, 21.79% (34/156) had bacterial coinfections, and 16.67% (26/156) were coinfected with other respiratory viruses.The most frequently isolated bacteria were Haemophilus influenzae (8.33%, 13/156) and Klebsiella pneumoniae (4.49%, 7/156), while respiratory syncytial virus (RSV) and Parainfluenza were the most common viruses isolated (5.77%, 9/156). There was no statistical significance in the co-infection between the two groups (P>0.05).
Table 2
| Characteristics | Conventional treatments | P value | ||
|---|---|---|---|---|
| Total (N=156) | Survivor group (N=142) | Death group (N=14) | ||
| Laboratory index | ||||
| PO2 (kPa) | 9.35±2.12 | 10.29±2.47 | 8.61±3.66 | 0.04* |
| PCO2 (kPa) | 5.13±2.08 | 4.82±0.73 | 6.58±2.30 | 0.02* |
| PaO2/FiO2 (P/F) | 239.21±98.81 | 250.28±80.99 | 109.77±79.58 | <0.001* |
| WBC (×109/L) | 8.05±5.11 | 8.55±5.21 | 7.12±4.71 | 0.36 |
| Monocyte (×109/L) | 0.49±0.41 | 0.52±0.53 | 0.34±0.42 | 0.06 |
| Lymphocyte (×109/L) | 2.48±1.75 | 2.75±1.88 | 2.21±1.58 | 0.26 |
| Hemoglobin (g/L) | 104.32±15.58 | 105.18±15.75 | 96.64±21.93 | 0.03* |
| Lactate dehydrogenase (U/L) | 1,012.54±789.95 | 950.36±809.38 | 1,677.14±1,004.02 | <0.001* |
| Serum albumin (g/L) | 32.65±5.45 | 33.29±5.78 | 28.89±6.08 | <0.001* |
| C-reactive protein (mg/L) | 32.78±38.40 | 34.29±41.55 | 27.25±26.03 | 0.89 |
| PCT (ng/mL) | 6.38±13.85 | 4.96±15.28 | 8.55±20.64 | 0.07 |
| ALT (U/L) | 40.14±42.65 | 37.18±41.84 | 49.79±36.32 | 0.01* |
| AST (U/L) | 112.38±110.75 | 95.14±118.94 | 198.71±211.36 | <0.001* |
| CK-MB (U/L) | 32.67±20.75 | 29.32±18.96 | 46.50±29.16 | <0.001* |
| Radiological finding(s) | ||||
| X-ray/CT | ||||
| Consolidation (≤1 lobe) | 38 (24.36) | 37 (26.06) | 1 (7.14) | 0.12 |
| Consolidation (≥2 lobes) | 88 (56.41) | 75 (52.82) | 13 (92.86) | <0.001* |
| Pleural effusion | 76 (48.72) | 67 (47.18) | 9 (64.29) | 0.30 |
| Pneumothorax | 9 (5.77) | 7 (4.93) | 2 (14.29) | 0.15 |
| Co-infections | ||||
| Adenovirus, virus | 26 (16.67) | 23 (16.20) | 3 (21.43) | – |
| Respiratory syncytial virus | 9 (5.77) | 7 (4.93) | 2 (14.29) | 0.15 |
| Parainfluenza | 9 (5.77) | 8 (5.63) | 1 (7.14) | >0.99 |
| FA/FB | 4 (2.56) | 4 (2.82) | 0 (0.00) | 0.52 |
| Rhinovirus | 4 (2.56) | 4 (2.82) | 0 (0.00) | 0.52 |
| Adenovirus, bacteria | 34 (21.79) | 27 (19.01) | 7 (50.00) | – |
| Haemophilus influenzae | 13 (8.33) | 11 (7.75) | 2 (14.29) | 0.74 |
| Klebsiella pneumoniae | 7 (4.49) | 6 (4.23) | 1 (7.14) | 0.64 |
| Staphylococcus aureus | 6 (3.85) | 4 (2.82) | 2 (14.29) | 0.16 |
| Acinetobacter baumannii | 3 (1.92) | 2 (1.41) | 1 (7.14) | 0.64 |
| Pseudomonas aeruginosa | 4 (2.56) | 3 (2.11) | 1 (7.14) | 0.80 |
| Escherichia coli | 1 (0.64) | 1 (0.70) | 0 (0.00) | >0.99 |
| Adenovirus, Mycoplasma pneumoniae | 53 (33.97) | 50 (35.21) | 3 (21.43) | 0.30 |
Continuous variables are presented as mean ± standard deviation and categorical variables are presented as n (%). *, statistically significant. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK-MB, creatine kinase-MB; CT, computed tomography; FA, fluorescein angiography; FB, foreign body; PCO2, partial pressure of carbon dioxide; PCT, procalcitonin; PO2, partial pressure of oxygen; SAP, severe adenovirus pneumonia; WBC, white blood cell.
Treatments, complications, and outcomes
The treatments administered to the 156 patients with SAP are shown in Table 3. Most patients received immunoglobulin therapy (94.87%, 148/156) and antibiotic therapy (89.74%, 140/156). Corticosteroid therapy was used in 65.38% (102/156) of cases. Additionally, 42.95% (67/156) underwent bronchoscopy combined with bronchoalveolar lavage (BAL). Oxygen supplement was needed in 27.56% (43/156), non-invasive MV was required in 5.13% (8/156), invasive MV was required in 15.38% (24/156) of patients, and 6.41% (10/156) underwent continuous renal replacement therapy (CRRT). Table 3 also outlines the patients’ complications and outcomes. The most common complication was respiratory failure (18.59%, 29/156), followed by septic shock (7.05%, 11/156), and ARDS (6.41%, 10/156). A total of 32 patients (20.51%) were admitted to the pediatric intensive care unit (PICU), with a mortality rate of 43.75% (14/32). Overall, 14 patients (8.97%) died. The first cause of death was septic shock (10/14,71.43%), followed by ARDS (8/14, 57.14%). The median length of hospitalization was 19.78±14.08 days.
Table 3
| Characteristics | Conventional treatments | P value | ||
|---|---|---|---|---|
| Total (N=156) | Survivor group (N=142) | Death group (N=14) | ||
| Treatments | ||||
| Drug therapy | ||||
| Immunoglobulin | 148 (94.87) | 135 (95.07) | 13 (92.86) | 0.72 |
| Antibiotics | 140 (89.74) | 126 (88.73) | 14 (100.00) | 0.18 |
| Corticosteroid | 102 (65.38) | 90 (63.38) | 12 (85.71) | 0.90 |
| Bronchoscopy | ||||
| Combined with bronchoalveolar lavage | 67 (42.95) | 59 (41.55) | 8 (57.14) | 0.26 |
| Oxygen supplement | 43 (27.56) | 43 (30.28) | 0 (0.00) | 0.02* |
| Non-invasive mechanical ventilation | 8 (5.13) | 8 (5.63) | 0 (0.00) | 0.36 |
| Invasive mechanical ventilation | 24 (15.38) | 10 (7.04) | 14 (100.00) | <0.001* |
| CRRT | 10 (6.41) | 2 (1.41) | 8 (57.14) | <0.001* |
| Complication | ||||
| Respiratory failure | 29 (18.59) | 15 (10.56) | 14 (100.00) | <0.001* |
| Septic shock | 11 (7.05) | 1 (0.7) | 10 (71.43) | <0.001* |
| ARDS | 10 (6.41) | 2 (1.41) | 8 (57.14) | <0.001* |
| Toxic encephalopathy | 9 (5.77) | 3 (2.11) | 6 (42.86) | <0.001* |
| Acute renal failure | 4 (2.56) | 0 (0.00) | 4 (28.57) | <0.001* |
| Pneumorrhagia | 3 (1.92) | 1 (0.7) | 2 (14.29) | 0.01* |
| Gastrointestinal hemorrhage | 3 (1.92) | 1 (0.7) | 2 (14.29) | 0.01* |
| Heart failure | 3 (1.92) | 0 (0.00) | 3 (21.43) | <0.001* |
| Liver failure | 2 (1.28) | 0 (0.00) | 2 (14.29) | <0.001* |
| Outcomes | ||||
| The length of hospitalization (days) | 19.78±14.08 | 15.83±8.30 | 23.43±23.19 | 0.33 |
| Dead | 14 (8.97) | 0 (0.00) | 14 (100.00) | <0.001* |
Categorical variables are presented as n (%) and continuous variables are presented as mean ± standard deviation. *, statistically significant. ARDS, acute respiratory distress syndrome; CRRT, continuous renal replacement therapy; SAP, severe adenovirus pneumonia.
Influencing factors of death in children with SAP undergoing conventional treatments
Univariate analysis of factors associated with death in children with SAP undergoing conventional treatments is presented in Tables 1-3. Table 1 shows demographics and clinical characteristics of the patients. Survivors and non-survivors did not differ significantly in terms of sex or age (all P values >0.05). Clinical characteristics such as shortness of breath, cyanosis, elevated heart rate, decreased SpO2, low blood pressure, and capillary refill time over 3 seconds differed significantly between groups (P<0.05). Table 2 shows significant differences in laboratory and radiological findings, including PO2, PCO2, lactate dehydrogenase (LDH), serum albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase-MB fraction (CK-MB), and pulmonary consolidation involving two or more lobes (P<0.05). Table 3 shows the treatments, complications and outcomes between these two groups. There were no differences in drug therapy (all P>0.05). There were significant differences in the need for oxygen supplement, invasive MV and CRRT between the two groups (P<0.05). Complications such as respiratory failure, septic shock, ARDS, and toxic encephalopathy were significantly more common in non-survivors (P<0.05).
Multivariate analysis of factors influencing death in children with SAP is shown in Table 4. Independent risk factors for death included decreased SpO2 [odds ratio (OR): 32.336, 95% confidence interval (CI): 2.385–619.473, P=0.02], increased pressure of carbon dioxide in arterial blood (PaCO2) (OR: 2.187, 95% CI: 1.079–4.434, P=0.03), and pulmonary consolidation affecting two or more lobes (OR: 9.071, 95% CI: 1.123–73.248, P=0.04).
Table 4
| Variables | β | P | OR | OR 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Decreased SpO2 | 2.133 | 0.02 | 32.336 | 2.385 | 619.473 |
| Increased PaCO2 | 0.782 | 0.03 | 2.187 | 1.079 | 4.434 |
| Lung consolidation (≥2 lobes) | 2.205 | 0.04 | 9.071 | 1.123 | 73.248 |
CI, confidence interval; OR, odds ratio; PaCO2, partial pressure of carbon dioxide; SpO2, peripheral oxygen saturation; SAP, severe adenovirus pneumonia.
Discussion
AdV infection is a leading cause of death in severe pneumonia, with mortality rates ranging from 3.4% to 16.7% in previous studies (3,20,21). When children with SAP progress to MODS, the mortality rate can exceed 50% (22). In our study, the overall mortality of SAP was 8.97%. However, in patients in whom conventional treatments failed in the PICU, the mortality rate was much higher at 43.75% (14/32). The first cause of death was ARDS (57.14%). This is consistent with previously reported literature. Most patients in the conventional treatment death group experienced severe complications such as respiratory failure (100%), septic shock (71.43%), and ARDS (57.14%). Aggressive conventional treatments, including immunomodulatory therapy, bronchoscopy, and respiratory support, appeared to be ineffective in these cases. All the 14 dead patients did not undergo ECMO therapy because of the rapid deterioration.
Previous studies have shown that children between 6 and 23 months of age are more susceptible to developing SAP (23,24). In our study, the median age of children with SAP was 30.12±28.78 months, slightly older than that reported in previous studies. In the conventional treatment death group, the mean age was 19.00±12.51 months. However, there was no significant difference in age between the conventional treatment death group and the survivor group. While clinical practice often focuses on critically ill infants around 1 year of age, our findings suggest that children older than 2 years should not be overlooked. In immunocompetent children with SAP, symptoms are often nonspecific. Most patients present with high fever, accompanied by cough and wheezing. In severe cases, symptoms escalate to systemic toxicity and respiratory distress, with other common symptoms such as diarrhea, abdominal pain and conjunctival congestion (7). In our study, severe respiratory symptoms, including shortness of breath, cyanosis, and decreased SpO2 were more prevalent in the conventional treatment death group, whereas systemic symptoms like vomiting, diarrhea, and seizures were less prominent. The development of these severe respiratory symptoms is attributed to the excessive release of inflammatory cytokines due to AdV infection, leading to alveolar epithelial and endothelial cell apoptosis, alveolar epithelial barrier destruction, vascular leak, alveolar edema, hypoxia, and, in severe cases, ARDS, the mechanism is similar to that when COVID infects the lungs (25).
Pulmonary consolidation and atelectasis are characteristic imaging findings in SAP (10). As pneumonia progresses, imaging often reveals increasing involvement of lung lobes (7). In our study, 92.86% of patients in the conventional treatment death group had pulmonary consolidation in two or more lobes, accompanied by markedly decreased SpO2 and elevated PaCO2. We also identified decreased SpO2, increased PaCO2, and pulmonary consolidation (involving two or more lobes) as independent risk factors for death in immunocompetent children with SAP undergoing conventional treatments. Pulmonary consolidation and atelectasis occur due to the infection and injury of type I and type II alveolar epithelial cells by AdV. This results in increased exudation, impaired gas diffusion, and reduced pulmonary surfactant secretion (7), ultimately causing hypoxemia and carbon dioxide retention. Bronchoscopy combined with BAL has been identified as an effective therapy for alleviating pulmonary consolidation and atelectasis (26). In our study, 42.95% (67/156) of patients underwent bronchoscopy combined with BAL, including 57.14% (8/14) in the conventional treatment death group. Six patients in the death group did not undergo bronchoscopy due to their unstable condition, which rendered them unable to tolerate the procedure. However, our study found no significant difference in the use of bronchoscopy combined with BAL between the conventional treatment death group and the survivor group. This is in line with our previously published study, which demonstrated that early BAL in severe cases may not shorten the disease course or improve prognosis (16). Similarly, MV is an effective therapy for managing hypoxemia and carbon dioxide retention, but in our study, 100% patients in the conventional treatment death group received MV therapy, and their condition continued to deteriorate. A possible explanation is that AdV DNA released by lysed cell is recognized by immune cells, triggering the release of large quantities of cytokines and chemokines. This causes an intense “inflammatory storm” in patients with high AdV loads, particularly in the conventional treatment death group (7). Therefore, physical therapies like bronchoscopy combined with BAL and MV therapy do not effectively improve the condition because of the continued release of these inflammatory mediators. Previous studies have identified the “inflammatory storm” as a critical mechanism in SAP (27,28). Unfortunately, immunomodulatory therapies, including intravenous immunoglobulin and glucocorticoids which have anti-inflammatory, immunomodulatory, and anti-shock properties also failed to show efficacy in some patients within the conventional treatment death group. When conventional treatments are ineffective, monoclonal antibodies targeting cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) may offer potential therapeutic strategies. However, their safety and efficacy in viral infections remain uncertain (29,30).
ECMO, which offers low oxygen flow and small tidal volumes, reduces ventilator-induced lung injury and promotes lung recovery. It may benefit patients with SAP who do not respond to MV therapy. In our previously published study involving 19 patients receiving ECMO for SAP, the overall mortality rate was 7.44%, and the PICU mortality rate was 34.2% (15). In comparison, the current study, which excluded ECMO patients, revealed a higher overall mortality (8.97%) and a higher PICU mortality (43.75%). These differences indicate that ECMO can significantly benefit patients with SAP. Based on this and our previous findings, we recommended ECMO should be considered early when patients with decreased SpO2, increased PaCO2, and lung consolidation (involving two or more lobes), especially if conventional treatments fail to reverse the deterioration. However, ECMO use carries risks such as bleeding, infection, pneumothorax, and an associated increase in mortality (15); thus, vigilance is required.
Limitations
There are several limitations in our study. First, we did not assess key inflammatory markers such as ferritin, cytokines, and chemokines. Second, we did not investigate the specific AdV serotypes, which may influence the severity of SAP. Lastly, this was a single-center retrospective study, which may limit the generalizability of our findings.
Conclusions
In conclusion, the conventional treatment death group exhibited more severe respiratory symptoms. Decreased SpO2, increased PaCO2, and lung consolidation (≥2 lobes) were identified as independent risk factors for death in immunocompetent children with SAP receiving conventional treatments. We deduce that in cases where patients present with decreased SpO2, elevated PaCO2, and extensive lung consolidation, conventional treatments may not halt disease progression. In conjunction with our previous study, when these factors are present, and conventional treatments fail to halt the clinical decline, ECMO may need to be considered as soon as possible.
Acknowledgments
The authors would like to thank all patients and their families involved in this study.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-184/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-184/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-184/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-184/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committee of the Guangzhou Women and Children’s Medical Center at Guangzhou Medical University (2024NO.404A01) and individual consent for this retrospective analysis was waived
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee J, Choi EH, Lee HJ. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991-2007). J Med Virol 2010;82:624-31. [Crossref] [PubMed]
- Wu PQ, Zeng SQ, Yin GQ, et al. Clinical manifestations and risk factors of adenovirus respiratory infection in hospitalized children in Guangzhou, China during the 2011-2014 period. Medicine (Baltimore) 2020;99:e18584. [Crossref] [PubMed]
- Shi J, Zhou Y, Wang F, et al. A case series of children with adenovirus pneumonia: three-year experiences in a tertiary PICU. BMC Pediatr 2020;20:375. [Crossref] [PubMed]
- Hakim FA, Tleyjeh IM. Severe adenovirus pneumonia in immunocompetent adults: a case report and review of the literature. Eur J Clin Microbiol Infect Dis 2008;27:153-8. [Crossref] [PubMed]
- Lynch JP 3rd, Kajon AE. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin Respir Crit Care Med 2016;37:586-602. [Crossref] [PubMed]
- Echavarría M. Adenoviruses in immunocompromised hosts. Clin Microbiol Rev 2008;21:704-15. [Crossref] [PubMed]
- Zhang J, Zhu Y, Zhou Y, et al. Pediatric adenovirus pneumonia: clinical practice and current treatment. Front Med (Lausanne) 2023;10:1207568. [Crossref] [PubMed]
- National Health Commission of the People's Republic of China State Administration of Traditional Chinese Medicine. Diagnosis and Treatment of children with adenovirus pneumonia (2019 edition). Chinese Journal of Clinical Infectious Diseases 2019;161-6.
- Nunes-Silva C, Vilares AT, Schweitzer V, et al. Non-COVID-19 respiratory viral infection. Breathe (Sheff) 2022;18:210151. [Crossref] [PubMed]
- Chen X, Lv J, Qin L, et al. Severe Adenovirus Pneumonia Requiring Extracorporeal Membrane Oxygenation Support in Immunocompetent Children. Front Pediatr 2020;8:162. [Crossref] [PubMed]
- Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015;16:428-39. [Crossref] [PubMed]
- Ramanathan K, Tan CS, Rycus P, et al. Extracorporeal Membrane Oxygenation for Severe Adenoviral Pneumonia in Neonatal, Pediatric, and Adult Patients. Pediatr Crit Care Med 2019;20:1078-84. [Crossref] [PubMed]
- Lee M, Kim S, Kwon OJ, et al. Treatment of Adenoviral Acute Respiratory Distress Syndrome Using Cidofovir With Extracorporeal Membrane Oxygenation. J Intensive Care Med 2017;32:231-8. [Crossref] [PubMed]
- Xu XH, Fan HF, Shi TT, et al. Analysis of mortality risk factors in children with severe adenovirus pneumonia: A single-center retrospective study. Pediatr Neonatol 2023;64:280-7. [Crossref] [PubMed]
- Shi T, Chen C, Fan H, et al. Impact of extracorporeal membrane oxygenation in immunocompetent children with severe adenovirus pneumonia. BMC Pulm Med 2023;23:41. [Crossref] [PubMed]
- Xu XH, Fan HF, Shi TT, et al. Influence of the timing of bronchoscopic alveolar lavage on children with adenovirus pneumonia: a comparative study. BMC Pulm Med 2021;21:363. [Crossref] [PubMed]
- Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011;66:ii1-23. [Crossref] [PubMed]
- Oliveira FRC, Macias KM, Rolli PA, et al. Management of acute respiratory distress syndrome in a child with adenovirus pneumonia: case report and literature review. Rev Paul Pediatr 2020;38:e2018280. [Crossref] [PubMed]
- Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2-8. [Crossref] [PubMed]
- Bakir J, Juárez MDV, Lución MF, et al. Clinical and epidemiological study of acute lower respiratory tract infections caused by adenovirus in hospitalized children. Nineteen years of active epidemiological surveillance. Arch Argent Pediatr 2020;118:193-201. [Crossref] [PubMed]
- Xu N, Chen P, Wang Y. Evaluation of Risk Factors for Exacerbations in Children with Adenoviral Pneumonia. Biomed Res Int 2020;2020:4878635. [Crossref] [PubMed]
- Chowdhury F, Shahid ASMSB, Ghosh PK, et al. Viral etiology of pneumonia among severely malnourished under-five children in an urban hospital, Bangladesh. PLoS One 2020;15:e0228329. [Crossref] [PubMed]
- Dai H, Xi H, Huang L, et al. Molecular Epidemiology and Clinical Features Analysis of Respiratory Adenovirus Infections Reveals Correlations between Genotype, Inflammatory Biomarkers, and Disease Severity. Biomed Res Int 2020;2020:4357910. [Crossref] [PubMed]
- Xie L, Zhang B, Zhou J, et al. Human adenovirus load in respiratory tract secretions are predictors for disease severity in children with human adenovirus pneumonia. Virol J 2018;15:123. [Crossref] [PubMed]
- Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev 2020;53:38-42. [Crossref] [PubMed]
- Lou Q, Zhang SX, Yuan L. Clinical analysis of adenovirus pneumonia with pulmonary consolidation and atelectasis in children. J Int Med Res 2021;49:300060521990244. [Crossref] [PubMed]
- Atasheva S, Shayakhmetov DM. Cytokine Responses to Adenovirus and Adenovirus Vectors. Viruses 2022;14:888. [Crossref] [PubMed]
- Cook J, Radke J. Mechanisms of pathogenesis of emerging adenoviruses. F1000Res 2017;6:90. [Crossref] [PubMed]
- Baslılar S, Pehlivan O. Evaluation of factors affecting the frequency and clinical course of COVID-19 in patients using anti-TNF-alpha agents. Rev Assoc Med Bras (1992) 2021;67:1286-92. [Crossref] [PubMed]
- Palacios Y, Chavez-Galan L. Immunosuppressant Therapies in COVID-19: Is the TNF Axis an Alternative? Pharmaceuticals (Basel) 2022;15:616. [Crossref] [PubMed]


