
Association between platelet-to-lymphocyte ratio and blood urea nitrogen levels in pediatric populations: evidence from the NHANES (2009–2018)
Highlight box
Key findings
• This study identified a significant negative correlation between platelet-to-lymphocyte ratio (PLR) and blood urea nitrogen (BUN) levels in children aged 12–18 years (P<0.001), which remained robust after adjustment for confounding variables. A non-linear negative association was identified, with an inflection point at PLR =118.45, beyond which the decline in BUN levels became more pronounced.
What is known and what is new?
• This study is the first to report a significant negative association between PLR and BUN in a pediatric population.
• A non-linear negative relationship was identified, with a critical threshold at PLR =118.45, consistent across subgroups defined by sex, race, and body mass index.
What is the implication, and what should change now?
• A significant non-linear negative association was found between PLR and BUN, suggesting that PLR may serve as an early biomarker for the prediction and prevention of kidney disease risk in children.
Introduction
As one of the main metabolites in the urea cycle, blood urea nitrogen (BUN) reflects the dynamic balance of glomerular filtration function and protein catabolism (1-3). Under normal physiological conditions, BUN is synthesized in the liver and excreted by the kidneys. However, when kidney function is impaired or protein metabolism is abnormal, BUN levels may fluctuate, making it a crucial marker for assessing renal function (4). Similarly, abnormal BUN levels in children may indicate early signs of kidney dysfunction or other metabolic disturbances. For instance, studies have demonstrated a correlation between elevated BUN levels and the development of acute kidney injury in children, suggesting its potential as a predictive marker of disease progression (5,6). BUN levels reflect the nutritional and metabolic status of children. Excessive protein intake may lead to prerenal azotemia, resulting in elevation BUN levels (7,8). Therefore, monitoring and studying BUN levels is essential in children for the timely diagnosis and management of related health conditions.
In clinical practice, the complete blood count (CBC) is a readily available and cost-effective test that provides rapid diagnostic results. More importantly, various indices and ratios derived from CBC, particularly the platelet-to-lymphocyte ratio (PLR), have been established as reliable inflammatory markers. In recent years, PLR has been widely used as an inflammation marker due to its availability from CBC panels, rapid results, and being inexpensive. Research has found that elevated PLR levels are commonly observed in patients with chronic inflammatory diseases, such as rheumatic disorders, diabetes, cardiovascular disorders, cancer, and autoimmune diseases, further highlighting the clinical value of PLR in assessing inflammatory status (9-11).
Although the importance of PLR and BUN in adult health research is widely recognized, little is known about the interaction and impact of these indicators in children. It is well known that children have significant differences in physiological characteristics from adults, which makes adult research results not directly applicable to children (12). For example, the functional activity of the urinary and immune systems during adolescence may influence PLR and BUN levels and their correlation in various ways (13,14). While some studies have explored the relationship between CBC-derived markers of inflammation and BUN levels, the results remain inconclusive (15-17). Notably, no systematic studies have examined the important predictive and prognostic value of PLR in detecting BUN levels in children. Therefore, we utilized a sample of children aged 12–18 years from the National Health and Nutrition Examination Survey (NHANES) to evaluate the relationship between PLR and BUN levels in children, addressing this research gap. We present this article in accordance with the STROBE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-193/rc).
Methods
Study population
The NHANES is a population-based survey conducted by the U.S. Centers for Disease Control and Prevention, which collects health and nutrition data from a nationally representative sample of the civilian, non-institutionalized U.S. population (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx) (18). It includes both healthy individuals and those with various chronic or acute health conditions. For the purposes of this study, we applied specific inclusion and exclusion criteria to focus on relatively healthy adolescents by removing participants with abnormal platelet counts, elevated creatinine levels, and other indicators of significant pathology. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments.
This study utilized data from 2009 to 2018. According to the World Health Organization’s definition, adolescents and young people are defined as individuals aged 10–19 years (19). Starting with 34,042 eligible individuals, we excluded 2,498 with missing BUN data and 26,616 who were either ≥19 or <10 years. However, further screening revealed a significant number of missing key indicators (such as BUN, PLR, and some covariates) in the 10–11-year age group in the NHANES database, and there were significant differences in physiological characteristics between individuals in this age group and those in the middle and late stages of puberty (12–18 years old). Further exclusions were made for participants with missing PLR or covariate data (n=2,842). Ultimately, 2,086 participants aged 12–18 years were included in the study (Figure 1).
Detection and definition of PLR and BUN
Blood samples were collected from participants via venipuncture. After undergoing standardized processing and storage procedures, the samples were sent to the National Center for Environmental Health at the Centers for Disease Control and Prevention for analysis. The concentration of BUN was quantitatively measured using the enzymatic conductivity rate method on the DxC 800 biochemical analyzer (Beckman Coulter Diagnostics, Brea, CA, USA). Additionally, CBC was performed with an automated hematology analyzer (Beckman Coulter DxH 800 analyzer, Beckman Coulter Diagnostics, Brea, CA, USA). PLR was calculated by dividing the platelet count by the lymphocyte count (20). The accuracy and dependability of these measurements were guaranteed by the rigorous quality control procedures employed in the NHANES laboratory methods.
Covariates
Based on current literature and clinical insights, we have included several potential confounding variables that may affect the relationship between PLR and BUN. These confounding variables include age, sex, race, poverty income ratio (PIR), body mass index (BMI, kg/m2), triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (15). Within the NHANES survey framework, we categorized ethnicity into five groups: Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other race (21). BMI was categorized as <25 and ≥25 kg/m2.
Statistical analysis
NHANES data from 2009 to 2018 were used for analysis. Within this cross-sectional study, we classified PLR into four categories, and subjects were categorized based on the quartiles of PLR. Continuous variables were presented as mean ± standard deviation (SD), and categorical variables were described by frequencies (%). To elucidate the relationship between PLR and BUN, linear regression models were established with the lowest quartile (Q1) as the reference group. In Model 1, no adjustments for covariates were made. Model 2 introduced adjustments for age, sex, and race. Model 3 expanded these adjustments to include age, sex, race, PIR, BMI, TC, TG, LDL-C, and HDL-C. Moreover, we performed an restricted cubic spline (RCS) analysis of PLR and BUN with adjusting for age and other covariates in Model 3. This approach provides more robust and visually interpretable results. Finally, we conducted subgroup analyses to assess the potential impact of sex, race, and BMI on the association between PLR and BUN. All statistical analyses were performed using R 4.4.0, with P<0.05 considered statistically significant.
Results
Baseline characteristics of the study participants
A total of 2,086 individuals were enrolled in this study, comprising 51.49% males and 48.51% females. The baseline characteristics of the study participants according to PLR quartiles are presented in Table 1. The PLR quartiles were defined as follows: Q1 <96.79, Q2 96.79–<118.45, Q3 118.45–<144.57, and Q4 ≥144.57. Notably, compared to participants in the lowest quartile, BUN levels were significantly reduced in those in the highest quartile. Furthermore, statistically significant differences were observed in age, sex, BMI, TC, and HDL-C across PLR quartiles (P<0.05).
Table 1
| Variables | Q1 (PLR <96.79) (N=522) | Q2 (96.79≤ PLR <118.45) (N=521) | Q3 (118.45≤ PLR <144.57) (N=521) | Q4 (PLR ≥144.57) (N=522) | P value |
|---|---|---|---|---|---|
| Age (years) | 15.20±2.01 | 15.02±1.93 | 14.76±1.96 | 14.72±1.97 | <0.001 |
| Sex | <0.001 | ||||
| Male | 308 (59.00) | 266 (51.06) | 263 (50.48) | 237 (45.40) | |
| Female | 214 (41.00) | 255 (48.94) | 258 (49.52) | 285 (54.60) | |
| Race | 0.84 | ||||
| Mexican American | 121 (23.18) | 123 (23.61) | 118 (22.65) | 105 (20.11) | |
| Other Hispanic | 51 (9.77) | 50 (9.60) | 49 (9.40) | 52 (9.96) | |
| Non-Hispanic White | 158 (30.27) | 153 (29.37) | 157 (30.13) | 147 (28.16) | |
| Non-Hispanic Black | 111 (21.26) | 124 (23.80) | 121 (23.22) | 143 (27.39) | |
| Other races, including multi-racial | 81 (15.52) | 71 (13.63) | 76 (14.59) | 75 (14.37) | |
| PIR | 2.03±1.53 | 2.01±1.51 | 2.10±1.53 | 2.01±1.49 | 0.74 |
| BMI (kg/m2) | 24.24±6.41 | 24.63±6.31 | 23.35±6.00 | 23.88±6.01 | 0.007 |
| <25 | 343 (65.71) | 328 (62.96) | 370 (71.02) | 344 (65.90) | 0.048 |
| ≥25 | 179 (34.29) | 193 (37.04) | 151 (28.98) | 178 (34.10) | |
| TG (mg/dL) | 155.76±28.82 | 153.92±28.57 | 153.26±28.30 | 155.63±28.68 | 0.39 |
| TC (mg/dL) | 80.17±51.44 | 75.18±43.61 | 72.66±41.93 | 68.59±39.57 | <0.001 |
| HDL-C (mg/dL) | 52.36±11.58 | 52.48±11.80 | 53.85±12.24 | 54.25±12.26 | 0.02 |
| LDL-C (mg/dL) | 87.36±25.80 | 86.41±24.97 | 84.84±24.88 | 87.66±24.45 | 0.26 |
| BUN (mg/dL) | 11.20±3.52 | 10.55±3.33 | 10.49±3.07 | 10.33±2.99 | <0.001 |
Data are presented as mean ± standard deviation for continuous variables and n (%) for categorical variables. BUN, blood urea nitrogen; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PLR, platelet-to-lymphocyte ratio; PIR, poverty income ratio; TC, total cholesterol; TG, triglyceride.
The association between PLR and BUN
We constructed three models adjusting for different covariates to evaluate the association between PLR and BUN (Table 2). The univariate linear regression model (Model 1) revealed a negative correlation between PLR and BUN (β=−1.25, 95% CI: −1.81, −0.69, P<0.001). This relationship remained in Model 2 (β=−0.92, 95% CI: −1.49, −0.34, P=0.002) and Model 3 (β=−1.03, 95% CI: −1.61, −0.45, P<0.001).
Table 2
| Variables | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | β (95% CI) | P value | |||
| Per 1 mg/dL BUN increase | −1.25 (−1.81 to −0.69) | <0.001 | −0.92 (−1.49 to −0.34) | 0.002 | −1.03 (−1.61 to −0.45) | <0.001 | ||
| PLR (categories) | ||||||||
| Q1 | Reference | Reference | Reference | |||||
| Q2 | −0.65 (−1.04 to −0.26) | 0.001 | −0.50 (−0.88 to −0.12) | 0.010 | −0.52 (−0.90 to −0.14) | 0.007 | ||
| Q3 | −0.72 (−1.11 to −0.32) | <0.001 | −0.53 (−0.91 to −0.14) | 0.007 | −0.54 (−0.92 to −0.16) | 0.005 | ||
| Q4 | −0.87 (−1.26 to −0.48) | <0.001 | −0.59 (−0.98 to −0.21) | 0.003 | −0.66 (−1.05 to −0.28) | <0.001 | ||
| P for trend | −0.27 (−0.39 to −0.14) | <0.001 | −0.18 (−0.30 to −0.06) | 0.004 | −0.20 (−0.32 to −0.08) | 0.001 | ||
Model 1: no covariates were adjusted; Model 2: adjusted for age, sex, and race; Model 3: adjusted for age, sex, race, PIR, BMI, TC, TG, LDL-C, and HDL-C. BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PLR, platelet-to-lymphocyte ratio; PIR, poverty income ratio; TC, total cholesterol; TG, triglyceride.
When PLR was divided into quartiles, with Q1 as the reference, the β coefficient for Q4 was significantly more negative than for Q1 (β=−0.87, 95% CI: −1.26, −0.48, P<0.001). After adjusting for all covariates, participants in the highest PLR quartile continued to show a negative correlation with BUN levels compared to those in the lowest quartile (β=−0.66, 95% CI: −1.05, −0.28, P<0.001).
Subgroup analysis and linear association between PLR and BUN
To assess the robustness of the relationship between PLR and BUN across various subgroups, we performed a subgroup analysis based on Model 3 (Table 3). The results indicated that in subgroups stratified by sex, race, and BMI, BUN levels showed a decreasing trend with increasing PLR. Furthermore, interaction tests revealed no significant interactions between sex, race, BMI, and PLR (P for interaction >0.05).
Table 3
| Subgroup variable | N | β (95% CI) | P value | P for interaction |
|---|---|---|---|---|
| Sex | 0.31 | |||
| Male | 1,074 | −0.01 (−0.01 to −0.01) | 0.001 | |
| Female | 1,012 | −0.01 (−0.99 to −0.01) | 0.048 | |
| Race | 0.07 | |||
| Mexican American | 467 | 0.00 (−0.00 to 0.01) | 0.51 | |
| Other Hispanic | 202 | −0.01 (−0.02 to 0.00) | 0.059 | |
| Non-Hispanic White | 615 | −0.01 (−0.02 to −0.01) | <0.001 | |
| Non-Hispanic Black | 499 | −0.01 (−0.01 to −0.01) | 0.03 | |
| Other races, including multi-racial | 303 | −0.01 (−0.01 to 0.00) | 0.28 | |
| BMI (kg/m2) | 0.80 | |||
| <25 | 1,385 | −0.01 (−0.99 to −0.01) | 0.008 | |
| ≥25 | 701 | −0.01 (−0.01 to −0.01) | 0.02 |
BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; PLR, platelet-to-lymphocyte ratio.
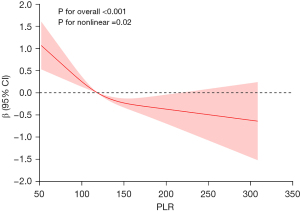
RCS analysis was employed to comprehensively demonstrate the relationship between PLR and BUN (Figure 2). The results showed a significant negative correlation between PLR and BUN overall (P for overall <0.001). Notably, we identified an inflection point at 118.45 after adjusting for covariates according to Model 3. The results indicate that when PLR is below the inflection point, the trend of BUN decreasing with increasing PLR is not significant. However, once PLR exceeds the inflection point, BUN exhibits a significant decline as PLR further increases. This suggests a significant non-linear relationship between PLR and BUN (P for non-linear =0.02).
Discussion
A previous study has shown that BUN levels are a commonly used indicator to evaluate renal function and systemic immune-inflammatory status (15). Moreover, variations in BUN are observed to be associated with a broad spectrum of conditions, including fluid excess, trauma, malnutrition, kidney disease, heart disease, and acute pancreatitis, all of which contribute to the initial assessment of children’s health status (22,23). In clinical practice, PLR is considered a representative marker of systemic inflammation and an important prognostic indicator for various cancers (24). It has the advantages of being cost-effective, rapid result reporting, and easily available from routine blood tests. Thus, PLR has become a part of routine clinical evaluation in recent years, especially in the diagnosis and risk monitoring of diseases in children, and has garnered increasing attention. For instance, 73 children diagnosed with inflammatory bowel disease (IBD) were included in a study analyzing the relationship between PLR and the severity of IBD in children. The study results showed that PLR was correlated with the diagnosis of IBD. Compared with 67 healthy subjects, the PLR was significantly higher in the patient group (25). Yang et al. evaluated 112 children with abdominal Henoch-Schonlein purpura in another study to explore the predictive value of PLR for treatment efficacy in the gastrointestinal bleeding group. Research has found that the PLR in the gastrointestinal bleeding group is significantly higher than that in the non-bleeding group, indicating that an increase in PLR could serve as a risk factor for gastrointestinal bleeding in children with Henoch-Schonlein purpura (26). Furthermore, in a retrospective study in Poland, PLR was recognized as a valuable indicator for identifying individuals at higher risk of systemic involvement in children with immunoglobulin A (IgA) vasculitis (27).
Our study confirmed the association between PLR and BUN levels, revealing a significant negative correlation. Moreover, this relationship was consistent across multiple regression models, enhancing its robustness. However, the underlying mechanisms have not yet been fully elucidated. One potential explanation for predicting BUN levels by PLR may involve the biological functions of platelets and lymphocytes. For example, several studies have reported that platelets play a key role in the pathogenesis and progression of urological diseases. Excessive stimulation of platelets, coupled with diminished antiplatelet regulation, may exacerbate platelet activation during acute kidney injury (28,29). Platelets can also induce the release of various cytokines and chemokines, such as IL-1β, platelet factor 4, and C-C chemokine ligand 5. These factors are capable of creating an inflammatory environment on the surface of renal vascular endothelial cells, thereby stimulating leukocyte recruitment and ultimately leading to renal injury (28,30). Moreover, lymphocytes, especially T cells as emerging mediators, are involved in the occurrence and recovery of kidney injury (31-33). Renal regulatory T cells may limit or prevent renal damage by releasing anti-inflammatory cytokines such as TGF-β and IL-10, while inhibiting the production of pro-inflammatory cytokines such as IL-1β, TNF-α, and IFN-γ (31,34).
In subgroup analysis, we observed that the relationship between PLR and BUN was consistent across various demographic factors, including sex, race, and BMI. This consistency indicates that the association is robust and independent of these variables. Furthermore, the uniformity across subgroups enhances the generalizability of our findings and underscores the potential clinical value of PLR as a non-invasive marker of renal function. Additionally, the non-linear negative association identified based on the RCS further illuminated the dynamic relationship between PLR and BUN. Specifically, when PLR was below the threshold of 118.45, the relationship between PLR and BUN was relatively weak, whereas when it exceeded this threshold, BUN levels decreased significantly as PLR increased. This finding implies that PLR may serve as a key indicator for assessing kidney health, especially in children with elevated PLR.
It has been reported that PLR tends to be elevated without a corresponding relationship with BUN levels in diabetic patients (35). By contrast, our findings suggest a negative correlation between PLR and BUN in healthy children. This discrepancy may stem from the different physiological states of the sample groups. In diabetic patients, the immune system is chronically stressed, resulting in an altered distribution and function of immune cells, which in turn leads to elevated PLR (36-38). This alteration in the immune state and the metabolic process of BUN appears to be independent of each other. However, adolescence is an important period of growth and development, during which the immune system continues to mature (39,40). BUN levels fluctuate with dietary protein intake, catabolism, and hydration status (15). Interestingly, the correlation between PLR and BUN levels observed in patients with preeclampsia is consistent with our findings (16). The interaction between increased platelet activation and lymphocyte responses in preeclampsia may similarly affect BUN levels.
Several strengths are significant in our research. Firstly, the study utilizes a nationally representative sample from NHANES, which reflects the racial and gender diversity of children and adolescents in the United States. The large sample size and appropriate covariate adjustments enhanced the reliability and representativeness of the findings. Secondly, this study comprehensively explored the effect of PLR on BUN levels in a pediatric population, addressing a gap in previous research and offering a novel perspective on childhood health assessment. Thirdly, our findings provide valuable insights into the existing knowledge base by elucidating the correlation between lower PLR levels and higher BUN levels. Nevertheless, there are several limitations in this study. To begin with, the retrospective cross-sectional design of this study makes the observations vulnerable to inherent selection bias. Hence, although we observed a correlation between PLR and BUN, we cannot establish whether a causal relationship exists between the two. Moreover, while we adjusted for multiple confounders in our regression analysis, other unmeasured or residual confounders may have influenced the results. Finally, this study does not fully cover children from different ethnic and socioeconomic backgrounds, which may limit the generalizability of the findings. Future studies should consider recruiting subjects from a broader range of populations to ensure their applicability and validity. While we acknowledge the potential of machine learning, our current analysis prioritized interpretability by using traditional regression and RCS models. We plan to explore machine learning in future research.
Conclusions
A significant non-linear negative relationship was found between PLR and BUN, indicating that it could serve as an early biomarker for prediction and prevention of kidney disease risk in children. However, the current findings cannot establish a causal relationship between the two; future large-scale prospective and experimental studies are required to confirm these results and elucidate the underlying mechanisms.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-193/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-193/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-193/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study used publicly available and de-identified data and was thus exempt from additional IRB review.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Veldhuisen DJ, Ruilope LM, Maisel AS, et al. Biomarkers of renal injury and function: diagnostic, prognostic and therapeutic implications in heart failure. Eur Heart J 2016;37:2577-85. [Crossref] [PubMed]
- Kirtane AJ, Leder DM, Waikar SS, et al. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J Am Coll Cardiol 2005;45:1781-6. [Crossref] [PubMed]
- Muller TL, Pluske JR, Plush KJ, et al. Serum creatinine is a poor marker of a predicted change in muscle mass in lactating sows. J Anim Physiol Anim Nutr (Berl) 2022;106:1009-16. [Crossref] [PubMed]
- Xu Z, Yue Y, Xu M, et al. The role of cut-off values for creatinine, blood urea nitrogen, and uric acid in prognostic assessment of chronic heart failure: a retrospective cohort study. BMC Cardiovasc Disord 2025;25:209. [Crossref] [PubMed]
- Fujita H, Shinjoh M, Ishii T, et al. Utility of fractional excretion of urea in the differential diagnosis of acute kidney injury in children. Pediatr Nephrol 2016;31:1349-53. [Crossref] [PubMed]
- Meena J, Yadav J, Kumar J, et al. Incidence, predictors, and short-term outcomes of acute kidney injury in children with diabetic ketoacidosis: a systematic review. Pediatr Nephrol 2023;38:2023-31. [Crossref] [PubMed]
- Arihan O, Wernly B, Lichtenauer M, et al. Blood Urea Nitrogen (BUN) is independently associated with mortality in critically ill patients admitted to ICU. PLoS One 2018;13:e0191697. [Crossref] [PubMed]
- Gary T, Pichler M, Schilcher G, et al. Elevated Blood Urea Nitrogen is Associated With Critical Limb Ischemia in Peripheral Arterial Disease Patients. Medicine (Baltimore) 2015;94:e948. [Crossref] [PubMed]
- Sarkar S, Kannan S, Khanna P, et al. Role of platelet-to-lymphocyte count ratio (PLR), as a prognostic indicator in COVID-19: A systematic review and meta-analysis. J Med Virol 2022;94:211-21. [Crossref] [PubMed]
- Verdoia M, Schaffer A, Barbieri L, et al. Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab 2015;41:304-11. [Crossref] [PubMed]
- Kriplani A, Pandit S, Chawla A, et al. Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and lymphocyte-monocyte ratio (LMR) in predicting systemic inflammatory response syndrome (SIRS) and sepsis after percutaneous nephrolithotomy (PNL). Urolithiasis 2022;50:341-8. [Crossref] [PubMed]
- Alderman EM, Breuner CCCommittee on Adolescence. Unique Needs of the Adolescent. Pediatrics 2019;144:e20193150. [Crossref] [PubMed]
- Charlton JR, Norwood VF, Kiley SC, et al. Evolution of the urinary proteome during human renal development and maturation: variations with gestational and postnatal age. Pediatr Res 2012;72:179-85. [Crossref] [PubMed]
- Li X, Gu L, Chen Y, et al. Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann Med 2021;53:1827-38. [Crossref] [PubMed]
- Guo C, Cai Q, Li Y, et al. A cross-sectional National Health and Nutrition Examination survey-based study of the association between systemic immune-inflammation index and blood urea nitrogen levels in United States adolescents. Sci Rep 2024;14:13248. [Crossref] [PubMed]
- Cui HX, Chen C, Jung YM, et al. Neutrophil-to-lymphocyte ratio (NLR) as a predictive index for liver and coagulation dysfunction in preeclampsia patients. BMC Pregnancy Childbirth 2023;23:4. [Crossref] [PubMed]
- Zeng P, Jiang C, Liu A, et al. Association of systemic immunity-inflammation index with metabolic syndrome in U.S. adult: a cross-sectional study. BMC Geriatr 2024;24:61. [Crossref] [PubMed]
National Center for Health Statistics 2025 . Available online: https://wwwn.cdc.gov/nchs/nhanes/Default.aspxWorld Health Organization 2025 . Available online: https://www.who.int/southeastasia/health-topics/adolescent-health- Wang L, Li X, Liu M, et al. Association between monocyte-to-lymphocyte ratio and prostate cancer in the U.S. population: a population-based study. Front Cell Dev Biol 2024;12:1372731. [Crossref] [PubMed]
- Wan Z, Guo J, Pan A, et al. Association of Serum 25-Hydroxyvitamin D Concentrations With All-Cause and Cause-Specific Mortality Among Individuals With Diabetes. Diabetes Care 2021;44:350-7. [Crossref] [PubMed]
- Liu H, Xin X, Gan J, et al. The long-term effects of blood urea nitrogen levels on cardiovascular disease and all-cause mortality in diabetes: a prospective cohort study. BMC Cardiovasc Disord 2024;24:256. [Crossref] [PubMed]
- Hantoosh SF, Al Jumaili FT, Zageer DS. Measurement of serum lipid profile parameters, blood urea nitrogen, serum uric acid and serum creatinine levels for children with acute lymphoblastic leukemia. Asian Journal of Biochemistry Genetics and Molecular Biology 2019;1:1-7.
- Ma L, Zhang Y, Shao Y, et al. Prognostic significance of systemic inflammatory response markers NLR, PLR, and MLR in advanced high-risk endometrial cancer following radiotherapy. Am J Cancer Res 2025;15:966-75. [Crossref] [PubMed]
- Zahmatkesh A, Sohouli MH, Hosseini SME, et al. The role of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in the diagnosis and severity of inflammatory bowel disease in children. Eur J Pediatr 2023;182:4263-70. [Crossref] [PubMed]
- Yang X, Lu R, Liu Q, et al. Analysis of the influencing factors of abdominal Henoch-Schonlein purpura in children with gastrointestinal bleeding and the clinical value of PLR. Am J Transl Res 2024;16:3867-74. [Crossref] [PubMed]
- Jaszczura M, Góra A, Grzywna-Rozenek E, et al. Analysis of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and mean platelet volume to platelet count ratio in children with acute stage of immunoglobulin A vasculitis and assessment of their suitability for predicting the course of the disease. Rheumatol Int 2019;39:869-78. [Crossref] [PubMed]
- Jansen MPB, Florquin S, Roelofs JJTH. The role of platelets in acute kidney injury. Nat Rev Nephrol 2018;14:457-71. [Crossref] [PubMed]
- Basile DP, Yoder MC. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc Hematol Disord Drug Targets 2014;14:3-14. [Crossref] [PubMed]
- Nair AS, Tauro L, Joshi HB, et al. Influence of homocysteine on regulating immunothrombosis: mechanisms and therapeutic potential in management of infections. Inflamm Res 2025;74:86. [Crossref] [PubMed]
- Dellepiane S, Leventhal JS, Cravedi P. T Cells and Acute Kidney Injury: A Two-Way Relationship. Front Immunol 2020;11:1546. [Crossref] [PubMed]
- Gharaie Fathabad S, Kurzhagen JT, Sadasivam M, et al. T Lymphocytes in Acute Kidney Injury and Repair. Semin Nephrol 2020;40:114-25. [Crossref] [PubMed]
- Cao C, Yao Y, Zeng R. Lymphocytes: Versatile Participants in Acute Kidney Injury and Progression to Chronic Kidney Disease. Front Physiol 2021;12:729084. [Crossref] [PubMed]
- Hao XM, Liu Y, Hailaiti D, et al. Mechanisms of inflammation modulation by different immune cells in hypertensive nephropathy. Front Immunol 2024;15:1333170. [Crossref] [PubMed]
- Wang JR, Chen Z, Yang K, et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol Metab Syndr 2020;12:55. [Crossref] [PubMed]
- Du J, Zhang W, Niu J, et al. Association between blood urea nitrogen levels and the risk of diabetes mellitus in Chinese adults: secondary analysis based on a multicenter, retrospective cohort study. Front Endocrinol (Lausanne) 2024;15:1282015. [Crossref] [PubMed]
- Falco G, Pirro PS, Castellano E, et al. The relationship between stress and diabetes mellitus. J Neurol Psychol 2015;3:7.
- Wilson JB, Epstein M, Lopez B, et al. The role of Neurochemicals, Stress Hormones and Immune System in the Positive Feedback Loops between Diabetes, Obesity and Depression. Front Endocrinol (Lausanne) 2023;14:1224612. [Crossref] [PubMed]
- Brenhouse HC, Schwarz JM. Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev 2016;70:288-99. [Crossref] [PubMed]
- Wood CL, Lane LC, Cheetham T. Puberty: Normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab 2019;33:101265. [Crossref] [PubMed]


