
LIN28B hypomethylation drives oncogenic signaling and stratifies poor prognosis in juvenile myelomonocytic leukemia
Highlight box
Key findings
• LIN28B-positive cases showed enrichment of cell cycle pathways and were correlated with high-methylation (HM), known risk factors (age >2 years, PTPN11 mutations), inferior 5-year overall survival (49.2% vs. 87.0%) and event-free survival (40.1% vs. 87.0%).
What is known and what is new?
• LIN28B is overexpressed in around 50% of juvenile myelomonocytic leukemia (JMML) cases but its epigenetic regulation and clinical utility are undefined.
• This study linked LIN28B activation to cell cycle dysregulation and high-risk clinical features, identifying it as a prognostic biomarker.
What is the implication, and what should change now?
• This study revealed LIN28B promoter hypomethylation and relevant LIN28B activation as a targetable high-risk JMML subtype with distinct molecular drivers. Further verifications should be carried out to confirm LIN28B’s regulatory effects on these targets.
Introduction
Juvenile myelomonocytic leukemia (JMML) is a rare, aggressive hematopoietic disorder classified as a myelodysplastic/myeloproliferative neoplasm. Predominantly affecting infants and young children, JMML is defined by dysregulated proliferation of myelomonocytic lineage cells (1,2). Oncogenic RAS pathway activation represents a central molecular driver in this disease (3). Given the aggressive clinical course of most JMML cases, allogeneic hematopoietic stem cell transplantation (HSCT) remains the sole potentially curative intervention (4). Consequently, comprehensive molecular characterization coupled with clinical parameter evaluation is essential for refining prognostic stratification in JMML patients (3,5,6).
Beyond established RAS signaling, dysregulation of embryonic regulatory programs contributes to leukemogenesis. LIN28B, an evolutionarily conserved oncofetal RNA-binding protein, modulates self-renewal capacity across embryonic, fetal, and malignant stem cell populations (7). Its functional importance is recognized in diverse stem-cell-derived malignancies, including neuroblastoma, acute myeloid leukemia (AML), and peripheral T-cell lymphoma. LIN28B is a highly conserved RNA-binding protein that plays a critical role in embryonic development, stem cell self-renewal, and metabolic regulation. It primarily regulates cell proliferation, differentiation, and tumorigenesis by inhibiting the maturation of the microRNA (miRNA) let-7 family (8). LIN28B binds to pre-let-7 and prevents its processing and maturation, thereby indirectly upregulating the activity of oncogenic signaling pathways. Additionally, LIN28B can directly bind to messenger RNA (mRNA) to regulate translation efficiency. The high expression of LIN28B leads to a significant downregulation of let-7 miRNA (e.g., let-7i). As a tumor suppressor, the loss of the let-7 family activates oncogenic signaling pathways (e.g., RAS/MYC), promotes tumor progression, and shortens patient survival.
Research indicates that approximately half of JMML patients exhibit high levels of LIN28B expression (9). Mechanistically, this overexpression is driven by a hypomethylated alternative promoter, relieving transcriptional repression. The hypomethylated new promoter region alleviates transcriptional repression, thereby promoting aberrant LIN28B expression. High LIN28B expression correlates with multiple clinical features, including older age at diagnosis, elevated hemoglobin F (HbF) levels, reduced platelet counts, PTPN11 gene mutations, multigene mutations, and a hypermethylation phenotype (10). These align with the adverse clinical prognostic factors in JMML. Patients with high LIN28B expression show significantly lower 5-year overall survival (OS) and event-free survival (EFS) rates compared to those with low expression, demonstrating poorer prognosis in terms of OS and survival without transplantation. Multivariate analysis identifies LIN28B overexpression as an independent risk factor influencing patient survival, suggesting its potential as a key therapeutic target in future strategies.
Despite emerging evidence linking LIN28B overexpression to adverse prognosis in JMML, the epigenetic mechanisms driving its dysregulation and its broader impact on molecular pathways remain poorly characterized. Furthermore, the clinical utility of LIN28B-associated methylation patterns for risk stratification has not been systematically explored. In this study, we integrate multi-omics analyses to delineate the role of LIN28B promoter hypomethylation in activating oncogenic signaling and stratifying high-risk JMML subgroups. By profiling LIN28B expression, methylation, and pathway alterations in a discovery cohort and validating findings in an independent cohort, we aim to establish LIN28B as both a mechanistic driver and a predictive biomarker, offering novel insights into JMML pathogenesis and precision therapy. We present this article in accordance with the STREGA reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-228/rc).
Methods
General information
This study is a retrospective analysis that collected clinical data from 86 JMML patients. Twenty-four JMML patients with paired methylation and RNA-sequencing data were recruited through the CHOPA001-JMML2020 trial (ChiCTR2000035471) from 2021 to 2022 by the China Hematology Oncology Pediatric Alliance (Table 1). Another validation cohort of 62 JMML patients with methylation and follow-up data was collected in the Department of Hematology and Oncology, Shanghai Children’s Medical Center (SCMC). EFS was defined as the time from diagnosis to the first major adverse event, including relapse after remission, failure to achieve remission, disease-related death, abandonment, development of a second malignancy, or transfer to other hospitals. OS was defined from diagnosis to death of any cause. All patients were diagnosed according to the 2016 JMML classification (1).
Table 1
| ID | Sex | Age (years) | MONO7 | HbF | Mutation | Type |
|---|---|---|---|---|---|---|
| JMML01 | Female | 0.7 | Negative | Elevated | CBL | Confirmed germline |
| JMML02 | Male | 5.9 | Negative | Normal | PTPN11 | Confirmed somatic |
| JMML03 | Female | 4.0 | Negative | Elevated | PTPN11 | Confirmed somatic |
| JMML04 | Male | 3.2 | NA | Elevated | PTPN11 | Confirmed somatic |
| JMML05 | Male | 0.9 | Negative | NA | PTPN11, KRAS | Not confirmed†, not confirmed† |
| JMML06 | Female | 4.2 | Negative | Normal | NRAS | Confirmed somatic |
| JMML07 | Female | 0.8 | Negative | Elevated | KRAS | Confirmed somatic |
| JMML08 | Male | 0.5 | NA | Elevated | PTPN11 | Confirmed somatic |
| JMML09 | Female | 1.8 | Negative | Elevated | PTPN11 | Confirmed somatic |
| JMML10 | Male | 2.5 | Negative | Elevated | PTPN11 | Confirmed somatic |
| JMML11 | Male | 3.1 | Negative | Elevated | PTPN11 | Confirmed somatic |
| JMML12 | Female | 1.1 | NA | NA | CBL | Confirmed germline |
| JMML13 | Male | 1.2 | Negative | Elevated | NRAS | Confirmed somatic |
| JMML14 | Female | 0.7 | Positive | Elevated | PTPN11 | Confirmed somatic |
| JMML15 | Male | 0.2 | Negative | Elevated | NRAS | Confirmed somatic |
| JMML16 | Male | 4.5 | Negative | Elevated | PTPN11 | Confirmed somatic |
| JMML17 | Female | 4.3 | Negative | Elevated | PTPN11 | Not confirmed |
| JMML18 | Female | 6.8 | NA | Elevated | PTPN11, NF1 | Confirmed somatic, not confirmed |
| JMML19 | Male | 3.0 | Negative | Elevated | PTPN11 | Confirmed somatic |
| JMML20 | Male | 4.8 | Negative | Elevated | PTPN11, NRAS, NF1, NF1, CBL | Confirmed somatic, confirmed somatic, not confirmed†, not confirmed†, not confirmed† |
| JMML21 | Male | 3.2 | Negative | Elevated | PTPN11 | Confirmed somatic |
| JMML22 | Male | 3.5 | NA | NA | PTPN11 | Confirmed somatic |
| JMML23 | Male | 5.8 | NA | Elevated | PTPN11, NRAS, NF1, NF1 | Confirmed somatic, confirmed somatic, confirmed somatic, not confirmed† |
| JMML24 | Male | 9.1 | Negative | Normal | KRAS | Confirmed somatic |
†, the mutations in these patients can not be verified because of the low variant allele frequency (VAF). HbF, hemoglobin F; JMML, juvenile myelomonocytic leukemia; NA, not available.
The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Institutional Review Board of Shanghai Children’s Medical Center (No. SCMCIRB-Y2020122). All participants’ legal guardians provided written informed consent.
Patient samples
All samples were obtained at the time of diagnosis and DNA was purified from bone marrow or peripheral blood. The elevated hemoglobin F (HbF) level was defined as follows: an HbF proportion exceeding 50% in patients in 2–4 months, exceeding 10% in 4–6 months, or exceeding 2% within 6 months (11).
Transcriptome sequencing (RNA-seq) analysis
Total RNA was extracted from bone marrow or peripheral blood of the JMML samples using TRIzol, and checked for RNA integrity number (RIN) (>6) to inspect RNA integrity. RNA-seq experiments were performed according to the manufacturer’s instructions. cDNA libraries were prepared using Illumina TruSeq Stranded Total RNA Kit. Purified cDNA libraries were sequenced on the Illumina HiSeq X Ten system (Illumina, United States) using PE 150 bp reads. RNA-seq data was aligned to human genome version GRCh37-lite with STAR (12) and duplicates marked using Picard. Gene transcription was analyzed using HTseq (13) and differential expression analysis was performed using the DEseq2 R package (14).
Identification of LIN28B expression
LIN28B expression status was determined by analyzing reads mapped to the LIN28B locus relative to the total aligned reads, with statistical significance assessed by Fisher’s exact test. A significance threshold of P<0.05 was applied, defining 14 out of 24 JMML patients meeting the minimum coverage requirement (≥5 reads mapped to LIN28B) as LIN28B expression-positive.
Genome-wide methylation analysis
Genomic DNA was bisulfite converted using the EZ DNA Methylation Kit (Zymo Research, California, USA) and processed on the Infinium MethylationEPIC Beadchip (Illumina Inc., Illumina, USA) following the manufacturer’s protocol. Raw data was processed using the champ.load function in the ChAMP R package (15) with the default parameters except for ‘filterNoCG = F’. All probes were then normalized using the champ.norm function with the BMIQ algorithm. Differentially methylated probes (DMPs) were identified using the champ.DMP function. Differentially methylated regions (DMRs) were identified using the champ.DMR function.
Annotation and enrichment analysis
Methylation probes were annotated using the IlluminaHumanMethylationEPICanno.ilm10b4.hg19 R package (16) and enrichment of genomic regions was calculated using HOMER version 4.11 (17). Gene Set Enrichment Analysis (GSEA) of RNA-seq was conducted using the ‘GSEA’ function of ClusterProfiler R package (18) with default parameters and referring to GO-BP (Gene Ontology Biological Process) and Kyoto Encyclopedia of Genes and Genomes (KEGG) genes from msigdbr (19) R package v7.5.1.
Hierarchical clustering of JMML samples
Hierarchical clustering was performed based on Manhattan distance and Ward’s linkage (ward. D2) using the pheatmap R package. We used the top 1,000 DMPs within the positive and negative groups of 24 JMML patients and clustered them into high and low methylation subgroups.
Statistical analysis
Fisher’s exact test was used to calculate the significance of the association between mutations and other categorized factors. The Kruskal-Wallis test was used for a difference between continuous variables. The Kaplan-Meier estimation and log-rank test were applied to the survival analysis. Unless otherwise stated, all P values were two-sided and values <0.05 were considered statistically significant.
Results
Differential methylation of LIN28B activated gene expression
LIN28B is not expressed in healthy individuals (8), while in our data, we stratified JMML patients into LIN28B-positive and LIN28B-negative groups based on Fisher’s exact test against the total aligned reads (Figure 1A). Notably, LIN28B expression was observed in over half of the JMML cohort (14/24, 58.3%). In addition to LIN28B, subsequent differential gene expression analysis (Figure 1B) revealed significant upregulation of the WT1 gene, a known oncogene overexpressed in AML (20). Mechanistically, WT1 enhances cellular proliferation by activating ERK pathway (21), a key downstream pathway of the RAS signaling pathway. Furthermore, transcriptional regulators such as POU1F1 (22) and GATA5 (23) were upregulated. In contrast, HEY1 gene (24), a transcription factor closely linked to Notch signaling, was significantly downregulated.
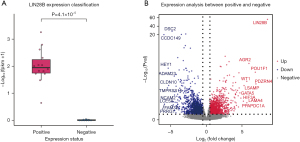
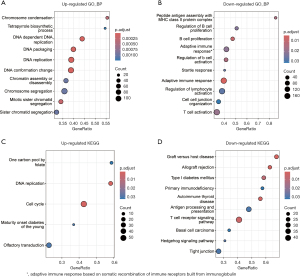
Next, we performed GO and KEGG pathway enrichment analyses on these differentially expressed genes (Figure 2). The results revealed significant upregulation of pathways related to DNA replication and cell cycle (Figure S1). Conversely, immune response pathways and lymphocyte activation processes showed significant downregulation in JMML patients in the LIN28B-positive group (Figure 2).
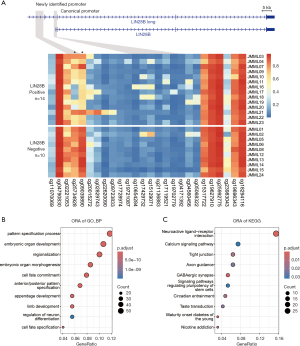
To explore methylation changes linked to differential gene expression, the same patient grouping strategy was employed for the paired methylation profiles of 24 JMML cases. Intriguingly, while both groups exhibited hypomethylation in canonical promoter regions, we found a DMR located approximately 5 kb upstream of the canonical promoter regions. Notably, the LIN28B-positive group showed significantly reduced methylation at this locus compared to the LIN28B-negative group (Figure 3A), which was reported as the newly identified promoter region of LIN28B gene (7). Among these, two probes showed a significant difference between the LIN28B-positive and -negative subgroups, and the low methylation of both probes was closely correlated with highly expressed LIN28B (Figure S2). Then we further identified the DMR between LIN28B-positive and -negative subgroups. Over-representation analysis (ORA) of DMRs displayed enrichment of pattern specification process and calcium signaling pathway (Figure 3B,3C).
Hierarchical clustering of DMPs
After identifying the methylation differences of LIN28B gene, we sought to determine the global methylation divergence between the two groups. By hierarchical clustering of the top 1,000 DMPs, the 24 JMML cases were re-stratified into high and low methylation subgroups (Figure 4). There were 75.3% (753/1,000) of probes exhibited significantly higher β-values in the high methylation subgroup compared to the low methylation subgroup, whereas 24.7% (247/1,000) probes showed the inverse pattern. Notably, the high methylation subgroup demonstrated strong concordance with LIN28B-positive status (Fisher’s exact test, P<0.001, Table 2). Furthermore, this subgroup was significantly enriched for previously reported adverse prognostic features (25), including age >2 years, PTPN11 mutations, and alignment with high/intermediate methylation subgroups identified by the JMML DNA methylation classifier (26). While the p-value of HbF levels did not reach significance, all patients in the high-methylation (HM) subgroup elevated HbF levels.
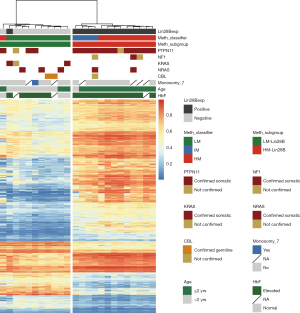
Table 2
| Index | Total (n=24) | HM (n=13) | LM (n=11) | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 15 (62.5) | 9 (69.2) | 6 (54.5) | 0.68 |
| Female | 9 (37.5) | 4 (30.8) | 5 (45.5) | |
| Age (years) | ||||
| Median (range) | 3.13 (0.18, 9.13) | 3.53 (1.75, 6.81) | 0.86 (0.18, 9.13) | 0.03* |
| <2 years | 9 (37.5) | 1 (7.7) | 8 (72.7) | 0.002* |
| ≥2 years | 15 (62.5) | 12 (92.3) | 3 (27.3) | |
| Mutations | ||||
| PTPN11 | 17 (70.8) | 13 (100.0) | 4 (36.4) | 0.001* |
| NF1 | 3 (12.5) | 3 (23.1) | 0 (0.0) | 0.22 |
| KRAS | 3 (12.5) | 0 (0.0) | 3 (27.3) | 0.08 |
| NRAS | 5 (20.8) | 2 (15.4) | 3 (27.3) | 0.63 |
| CBL | 3 (12.5) | 1 (7.7) | 2 (18.2) | 0.58 |
| MONO7 | ||||
| Positive | 1 (5.6) | 0 (0.0) | 1 (11.1) | >0.99 |
| Negative | 17 (94.4) | 9 (100.0) | 8 (88.9) | |
| HbF | ||||
| Elevated | 18 (85.7) | 12 (100.0) | 6 (66.7) | 0.06 |
| Normal | 3 (14.3) | 0 (0.0) | 3 (33.3) |
Data are presented as n (%), unless otherwise specified. *, P<0.05. DMPs, differentially methylated probes; HbF, hemoglobin F; HM, high methylation; JMML, juvenile myelomonocytic leukemia; LM, low methylation.
High methylation subgroup correlated with poor prognosis
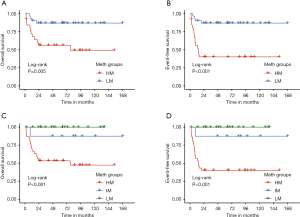
To validate the prognostic value of this methylation-based stratification, we applied the top 1,000 DMPs to an independent validation cohort of 62 JMML patients and performed clustering (Figure S3A and Table S1). The 62 JMML patients were grouped as 28 JMML patients with high methylation and 34 with low methylation. Prognostic analysis revealed that JMML patients in the HM subgroup exhibited significantly worse outcomes compared to the low methylation subgroup in both OS (49.2% vs. 87.0%) and EFS (40.1% vs. 87.0%) (Figure 5A,5B). When analysing the three subgroups divided by the JMML DNA methylation classifier, the low-methylation (LM) and intermediate-methylation (IM) subgroups showed similar methylation status (Figure S3B) and almost the same favorable outcome (Figure 5C,5D). Not surprisingly, the new IM subgroup mainly originated from the original LM subgroup (Figure S3C).
Discussion
Our study identifies LIN28B as a pivotal oncogenic driver in JMML, with its overexpression linked to promoter hypomethylation and aggressive disease biology. In contrast to healthy individuals where LIN28B is silenced (9), over half of JMML patients (58.3%) exhibited LIN28B activation, driven by hypomethylation of a newly identified upstream promoter region (7) (Figure 3). This epigenetic alteration likely alleviates transcriptional repression, mirroring mechanisms reported in other stem cell malignancies. The resultant LIN28B overexpression correlates with suppression of let-7 tumor suppressors, which may potentiate the RAS signaling pathway—a hallmark of JMML pathogenesis. Supporting this, we observed concurrent upregulation of WT1, a known RAS pathway activator in AML (27), and downregulation of HEY1, a Notch signaling modulator (24). These findings suggest that LIN28B activation synergizes with RAS dysregulation to disrupt differentiation and amplify proliferation, creating a permissive environment for leukemogenesis.
LIN28B overexpression drives tumorigenesis by forming an oncogenic axis that suppresses let-7 miRNA maturation, thereby derepressing critical oncogenes like MYC and RAS, which sustains stemness and metabolic reprogramming in cancers such as JMML (9). Importantly, several studies have put forward a classifier that stratifies JMML patients into distinct methylation subgroups with divergent clinical outcomes (10,26,28,29). In our research, we subdivided JMML patients into HM and LM subgroups with top 1,000 DMPs generated from LIN28B-positive and -negative subgroups, in which the HM subgroup was enriched for established adverse features such as older age (>2 years), PTPN11 mutations, and hypermethylation phenotypes in previously reported JMML DNA methylation classifier (26). Compared with the previously reported JMML DNA methylation classifier, our new methylation-based classification based on the 1000 DMPs reassigned most of the IM patients with favorable outcome as the LM subgroup. While under both classifications, the HM patients still exhibited significantly worse OS and EFS (Figure S3C and Figure 5). This aligns with prior studies linking DNA methylation patterns to JMML aggressiveness (10,26,28,29), but our work further identifies LIN28B as an essential epigenetic-expressional hub within this framework.
Limitations of our study include the modest cohort size for methylation analysis (n=24), which may limit the generalizability of our findings and reduce statistical power to detect subtle associations. Functional validation of LIN28B’s interaction with WT1 or HEY1 is also warranted. Future studies should employ functional assays to confirm LIN28B’s regulatory effects on these targets and elucidate its direct role in RAS pathway activation.
Conclusions
This study found LIN28B activation via a newly identified promoter hypomethylation and defined a high-risk JMML subgroup. These results position LIN28B as both a mechanistic hub and epigenetic biomarker for risk stratification.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the STREGA reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-228/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-228/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-228/prf
Funding: This study was supported by
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-228/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The study was approved by the Institutional Review Board of Shanghai Children’s Medical Center (No. SCMCIRB-Y2020122). All participants’ legal guardians provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391-405. [Crossref] [PubMed]
- Chang TY, Dvorak CC, Loh ML. Bedside to bench in juvenile myelomonocytic leukemia: insights into leukemogenesis from a rare pediatric leukemia. Blood 2014;124:2487-97. [Crossref] [PubMed]
- Bresolin S, Zecca M, Flotho C, et al. Gene expression-based classification as an independent predictor of clinical outcome in juvenile myelomonocytic leukemia. J Clin Oncol 2010;28:1919-27. [Crossref] [PubMed]
- Nishikawa T. Human Leukocyte Antigen-Haploidentical Haematopoietic Stem Cell Transplantation Using Post-Transplant Cyclophosphamide for Paediatric Haematological Malignancies. Cancers (Basel) 2024;16:600. [Crossref] [PubMed]
- Miao Y, Li B, Ding L, et al. PTPN11 mutation with additional somatic alteration indicates unfavorable outcome in juvenile myelomonocytic leukemia: a retrospective clinical study from a single center. Eur J Pediatr 2020;179:463-72. [Crossref] [PubMed]
- Hecht A, Meyer J, Chehab FF, et al. Molecular assessment of pretransplant chemotherapy in the treatment of juvenile myelomonocytic leukemia. Pediatr Blood Cancer 2019;66:e27948. [Crossref] [PubMed]
- Hovestadt V, Jones DT, Picelli S, et al. Decoding the regulatory landscape of medulloblastoma using DNA methylation sequencing. Nature 2014;510:537-41. [Crossref] [PubMed]
- Wang T, Wang G, Hao D, et al. Aberrant regulation of the LIN28A/LIN28B and let-7 loop in human malignant tumors and its effects on the hallmarks of cancer. Mol Cancer 2015;14:125. [Crossref] [PubMed]
- Helsmoortel HH, Bresolin S, Lammens T, et al. LIN28B overexpression defines a novel fetal-like subgroup of juvenile myelomonocytic leukemia. Blood 2016;127:1163-72. [Crossref] [PubMed]
- Murakami N, Okuno Y, Yoshida K, et al. Integrated molecular profiling of juvenile myelomonocytic leukemia. Blood 2018;131:1576-86. [Crossref] [PubMed]
- Huang SZ, Dunhua. Clinical Handbook of Pediatric Hematology. 3rd ed. Beijing: People's Medical Publishing House; 2010.
- Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15-21. [Crossref] [PubMed]
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31:166-9. [Crossref] [PubMed]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [Crossref] [PubMed]
- Tian Y, Morris TJ, Webster AP, et al. ChAMP: updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 2017;33:3982-4. [Crossref] [PubMed]
- Hansen KD. IlluminaHumanMethylationEPICanno.ilm10b4.hg19: Annotation for Illumina's EPIC methylation arrays. 2017.
10.18129/B9.bioc.IlluminaHumanMethylationEPICanno.ilm10b4.hg19 - Heinz S, Benner C, Spann N, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 2010;38:576-89. [Crossref] [PubMed]
- Yu G, Wang LG, Han Y, et al. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:284-7. [Crossref] [PubMed]
- Dolgalev I. msigdbr: MSigDB Gene Sets for Multiple Organisms in a Tidy Data Format. 2022.
- Lazzarotto D, Candoni A. The Role of Wilms' Tumor Gene (WT1) Expression as a Marker of Minimal Residual Disease in Acute Myeloid Leukemia. J Clin Med 2022;11:3306. [Crossref] [PubMed]
- Yang L, Han Y, Suarez Saiz F, et al. A tumor suppressor and oncogene: the WT1 story. Leukemia 2007;21:868-76. [Crossref] [PubMed]
- Seoane S, Martinez-Ordoñez A, Eiro N, et al. POU1F1 transcription factor promotes breast cancer metastasis via recruitment and polarization of macrophages. J Pathol 2019;249:381-94. [Crossref] [PubMed]
- Zheng R, Blobel GA. GATA Transcription Factors and Cancer. Genes Cancer 2010;1:1178-88. [Crossref] [PubMed]
- Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res 2008;102:1169-81. [Crossref] [PubMed]
- Niemeyer CM, Flotho C. Juvenile myelomonocytic leukemia: who's the driver at the wheel? Blood 2019;133:1060-70. [Crossref] [PubMed]
- Schönung M, Meyer J, Nöllke P, et al. International Consensus Definition of DNA Methylation Subgroups in Juvenile Myelomonocytic Leukemia. Clin Cancer Res 2021;27:158-68. [Crossref] [PubMed]
- Miwa H, Beran M, Saunders GF. Expression of the Wilms' tumor gene (WT1) in human leukemias. Leukemia 1992;6:405-9.
- Lipka DB, Witte T, Toth R, et al. RAS-pathway mutation patterns define epigenetic subclasses in juvenile myelomonocytic leukemia. Nat Commun 2017;8:2126. [Crossref] [PubMed]
- Stieglitz E, Mazor T, Olshen AB, et al. Genome-wide DNA methylation is predictive of outcome in juvenile myelomonocytic leukemia. Nat Commun 2017;8:2127. [Crossref] [PubMed]


