
Application of machine learning based on radiomics in the discrimination of intracranial germ cell tumours
Highlight box
Key findings
• Radiomics demonstrates outstanding diagnostic efficacy in distinguishing between germinomas and non-germinomatous germ cell tumours (NGGCTs).
What is known and what is new?
• Texture features can better detect the heterogeneity between germinomas and NGGCTs. Fine and medium textures can better reflect the microenvironment characteristics of germinoma.
• Multi-sequence magnetic resonance radiomics has good performance in differentiating germinomas from NGGCTs.
What is the implication, and what should change now?
• These findings make it possible to classify germ cell tumours histologically from imaging images.
Introduction
Intracranial germ cell tumours (ICGCTs) are relatively rare malignant tumours that primarily occur in children and adolescents, with the highest incidence in the second decade of life (1). They are usually located in the pineal region and suprasellar region, and can also occur in the basal ganglia, cerebellar hemisphere, or vermis. The incidence varies in different geographic regions, with ICGCTs accounting for 0.4–3.4% of primary central nervous system tumours in Western countries, while in Asia, especially Japan and Korea, the incidence is 5–8 times higher (2,3). The typical clinical manifestations include headache, nausea and vomiting, polydipsia, polyuria, visual impairment, decreased vision, Parinaud syndrome, and hemisensory disturbances.
In 2021, the World Health Organization central nervous system tumour classification categorised germ cell tumours based on histological types, including mature teratomas, immature teratomas, teratomas with somatic type malignant transformation, germinomas, embryonal carcinomas, yolk sac tumours, choriocarcinomas, and mixed germ cell tumours (4). Among these, germinomas are the most commonly seen, accounting for 50% to 70% of all cases (5,6). In terms of diagnosis, serum and cerebrospinal fluid alpha-fetoprotein (AFP) and beta human chorionic gonadotropin (β-HCG) are good laboratory indicators for distinguishing between germinomas and non-germinomatous germ cell tumours (NGGCTs) (7,8). According to previous clinical studies, the threshold of tumour markers varies in different continents. Hu et al. found that in Asia, it is relatively reasonable to use AFP >25 IU/L and β-HCG >50 ng/mL as diagnostic thresholds (9). Patients with markers above the threshold can be directly diagnosed as NGGCTs (10), while those below the threshold need pathological confirmation or diagnostic radiotherapy (11,12).
The treatment of germinomas and NGGCTs differs significantly. Germinomas are highly sensitive to both radiotherapy and chemotherapy. Calaminus et al. reported that the 5-year survival rate for patients with germinomas who received radiotherapy alone exceeded 90% (13). Additionally, an international multicenter collaborative study reported that 78% of 71 patients with intracranial germinomas achieved complete remission following chemotherapy alone (14). In contrast, the 5-year overall survival rate for NGGCTs with malignant components is typically lower than 70%, with the worst prognosis observed in tumours containing yolk sac tumours, choriocarcinomas, and embryonal carcinomas. These specific tumour types have a 5-year overall survival rate of less than 50% (15). For these patients, intensive chemotherapy combined with craniospinal radiotherapy is usually administered. Furthermore, if feasible, surgical efforts are made to remove any residual tumour lesions before completing the treatment regimen (10). Therefore, accurate diagnosis prior to treatment is of utmost importance.
Magnetic resonance imaging (MRI) has excellent soft tissue resolution, multi-plane imaging, non-ionising radiation, and non-contrast agent advantages, making it display significant value in the diagnosis of ICGCTs (16). However, in the imaging diagnosis report, it is usually difficult to provide specific recommendations for the histological type of ICGCTs, which limits its utility in treatment decision-making. Moreover, the diagnosis of ICGCTs cannot rely solely on MRI in patients with tumour markers below the diagnostic threshold which need biopsy or diagnostic radiotherapy. Nevertheless, biomedical images contain potential pathological and physiological information, and this information can be quantitatively transformed by radiomics methods. Unlike biopsy, which can only obtain partial histopathological information, biomedical images can provide a more comprehensive depiction of the overall expression of tumours (16). This enables the identification of histological types through imaging while overcoming the constraints of insufficient biopsy material. Therefore, this study aims to establish a diagnostic model for discriminating between germinomas and NGGCTs based on radiomics features and relevant clinical laboratory features using machine learning (ML) methods and explore its feasibility. The goal is to circumvent the risks related to biopsy and to make up for the diagnostic errors arising from sampling limitations. We present this article in accordance with the TRIPOD reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-210/rc).
Methods
Image acquisition
We retrospectively collected data from 175 patients diagnosed with ICGCTs in our hospital between August 2010 and July 2024. Of these, 10 patients diagnosed with mature teratomas, 19 patients without pre-treatment MRI plain and contrast-enhanced scans, and 5 patients with poor image quality were excluded. In the end, 141 patients were included in the study. The data set included 71 cases of germinomas and 70 cases of NGGCTs. Among all the cases, 111 cases (germinomas 56, NGGCTs 55) were from the main hospital district and 30 cases (germinomas 15, NGGCTs 15) were from the branch hospital district (Figure 1). This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. The Ethics Committee of the Children’s Hospital of Chongqing Medical University approved the study [(2023) No. 7]. Written informed consent was waived for this retrospective study by the Institutional Review Board.
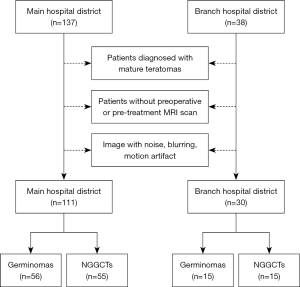
Inclusion criteria
- Histopathologically diagnosed ICGCTs.
- Patients with tumour markers (AFP >25 IU/L and/or β-HCG >50 ng/mL in the blood and/or cerebrospinal fluid) and imaging suggesting ICGCTs (17).
- Patients with diagnostic radiotherapy or diagnostic chemotherapy suggesting germinomas (18).
Exclusion criteria
- Patients diagnosed with mature teratomas.
- Patients without preoperative or pre-treatment MRI scan.
- Image with noise, blurring, and motion artefact.
All included patients underwent cranial MRI using either 1.5T or 3.0T scanners (Signa EXCITE HD, GE Healthcare, Chicago, IL, United States; Discovery MR750, GE Healthcare, Milwaukee, WI, United States; Achieva, Philips Healthcare, Best, the Netherlands). The scan parameters were as follows: (I) 1.5T MRI, T1-weighted imaging (T1WI), repetition time (TR) 2,000–3,000 ms, echo time (TE) 7–40 ms, slice thickness 6–6.5 mm; T2-weighted imaging (T2WI), TR 2,200–5,000 ms, TE 80–110 ms, slice thickness 6–6.5 mm; T2 fluid-attenuated inversion recovery (T2-FLAIR), TR 8,000 ms, TE 120–150 ms, slice thickness 6–6.5 mm. (II) 3.0T MRI, T1WI, TR 2,000–3,000 ms, TE 20–40 ms, slice thickness 6–6.5 mm; T2WI, TR 2,200–6,000 ms, TE 80–110 ms, slice thickness 6–6.5 mm; T2-FLAIR, TR 8,000 ms, TE 120–130 ms, slice thickness 6–6.5 mm. Furthermore, apparent diffusion coefficient (ADC) images are generated from the diffusion weighted imaging (DWI) sequence and only retained b=1,000 images in the DWI sequence to minimise the influence of T2 weighting. Following the intravenous administration of a gadolinium-based contrast agent at a dose of 0.1 mmol/kg, a gadolinium-enhanced T1WI was acquired in the axial plane. Ultimately, T1WI, T2WI, T2-FLAIR, gadolinium-enhanced T1WI, DWI (b=1,000), and ADC images were obtained in all patients included in the study. Image examples are illustrated in Figure 2.
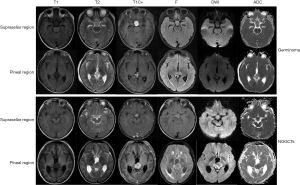
Image preprocessing
All patient images were imported into the research work platform of the picture archiving and communication system (PACS). To prevent variations in intensity features resulting from different scanning protocols, they were resampled to a voxel size of 3×3×3 mm3, underwent Z-score normalisation, and using a bin width of 5 standard intervals in order to perform grey-level discretization. The main analysis steps of radiomics are summarised in Figure 3.
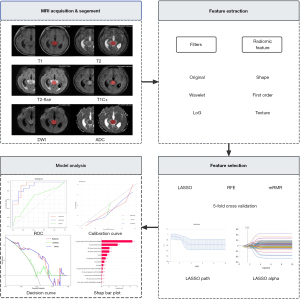
Tumour segmentation
In a double-blind setting, all images were segmented by two neuro-radiologists with a minimum of 5 years of experience using a research platform called uAI Research Portal (version 20240130, https://urp.united-imaging.com/) (19). A second neuro-radiologist with 15 years of experience examined the segmentations. During the delineation process, the components of haemorrhage, necrosis, and cystic changes were included in the segmentation, while efforts were made to minimise the inclusion of peritumoral oedema and surrounding blood vessel components. When multiple lesions were present in a patient, only the largest lesion was segmented.
Feature selection and model construction
The processes of feature extraction, feature selection and ML were performed using the uAI Research Portal. In this study, we extracted shape, first-order, texture, and Higher-order features [wavelet and Laplacian of Gaussian (LoG)]. The information of radiomics features is shown in Table S1. The features with intra-group correlation coefficients (ICCs) greater than 0.75 were considered to be highly reproducible and were therefore retained. The 111 patients from the main hospital district were randomly divided into a training set and a test set, with approximately 70% of the data allocated to the former and 30% to the latter. In the training set, we first implemented Z-score standardisation to eliminate dimensional discrepancies across features, thereby enhancing their comparability while mitigating the interference from extreme values. Then, the feature selection was performed in two stages. Initially, features exhibiting weak correlation and high repetition were excluded through the application of an analysis of variance and a Pearson correlation analysis. Feature selection was then repeated using least absolute shrinkage and selection operator (LASSO), minimum Redundancy Maximum Relevance (mRMR), and Recursive Feature Elimination (RFE). The most stable features were identified by 5-fold cross-validation.
Three classification models were used in this study, including logistic regression (LR), random forest (RF), and support vector machine (SVM). A total of nine optimised parameters models were established based on 5-fold cross-validation and used for internal testing. The area under the curve (AUC) values, accuracy, and Youden’s index (YI) were used to assess the difference in model diagnostic performance under and between different feature selection methods, and the best combination was reserved for external dataset testing.
Statistical analysis
Chi-squared test and t-test were used to analyse the demographic and clinical characteristics. P values of less than 0.05 were defined as significant and were retained to establish the clinical diagnostic model as a reference and establish the combined model.
Model interpretation
To assess the effectiveness of the ML models, we calculated several key metrics, including AUC, accuracy, sensitivity, specificity, precision, and F1 score. Then, we craft a columnar contrast plot to visually compare AUC values between the various models, using the Pyradiomics package (20) (https://pyradiomics.readthedocs.io/en/stable/) in Python 3.9.13. Receiver operating characteristic (ROC), calibration, and decision curves were used in the test cohort using RStudio (https://www.R-project.org/) (21). In addition, we used Shapley Additive exPlanations (SHAP) and Permutation importance to interpret the influence of the features on the final model. The Delong test was used to determine the differences in AUC between the clinical, multi-sequence, and clinical-multi-sequence radiomics models.
Results
Demographic and clinical characteristics
The demographic and clinical characteristics of the patients enrolled in the main hospital district are shown in Table 1. Statistical significance was observed in terms of age and headache.
Table 1
| Characteristics | Germinomas (n=56) | NGGCTs (n=55) | P value (<0.05) |
|---|---|---|---|
| Age (years) | 10.61±3.11 | 9.34±2.67 | 0.02 |
| Gender | 0.57 | ||
| Male | 39 (69.6) | 41 (74.5) | |
| Female | 17 (30.4) | 14 (25.5) | |
| Headache | 23 (41.0) | 34 (61.8) | 0.046 |
| Nausea and vomiting | 29 (51.8) | 36 (65.5) | 0.15 |
| Visual impairment | 11 (19.6) | 19 (34.5) | 0.12 |
| Polydipsia and polyuria | 22 (39.3) | 17 (31.0) | 0.36 |
| Poor appetite | 14 (25) | 7 (12.7) | 0.16 |
| Position | 0.28 | ||
| Pineal | 31 (55.4) | 33 (60.0) | |
| Suprasellar | 17 (30.4) | 19 (34.5) | |
| Bifocal | 8 (14.3) | 3 (5.5) |
Data are presented as mean ± standard deviation or n (%). NGGCTs, non-germinomatous germ cell tumours.
Radiomics feature selection, prediction model building and validation
A total of 6,039 features were extracted from the training set, and only 459 features were retained using ICC, analysis of variance, and Pearson correlation analysis. Then, based on 5-fold cross-validation, we retained 9, 10, and 10 features with voting counts greater than 3 or 4 under three different selection methods: LASSO, mRMR, and RFE, respectively.
A total of nine multi-sequence radiomics classification models were established under their respective hyperparameters (Table S2), based on the combination of multiple feature selection and classifiers (Table S3). The AUC values of the test set under each model were presented in the columnar contrast plot, as illustrated in Figure 4. In a comprehensive comparison of the performance of each model (Table S4), the combination test set of LASSO + RF and RFE + RF regression showed the most optimal performance, with AUC values of 0.823 and 0.821, and both accuracies of 0.758 and YI of 0.733. The aforementioned two combinations were retained, and the case data of the branch hospital district were used to conduct another test. The results demonstrated that LASSO + LR exhibited the optimal diagnostic performance, with the AUC of 0.804 (Table S4). The selected clinical characteristics were incorporated into the construction of a clinical-radiomics model and utilised to develop a clinical model. Both models were then subjected to external testing. The performances of the three classification model test sets were intuitively compared by ROC, decision curves, and calibration curves, as illustrated in Figure 5. The AUC values of the models were calculated, and the best sensitivity, specificity, accuracy, precision, and F1-score were recorded in Table 2.
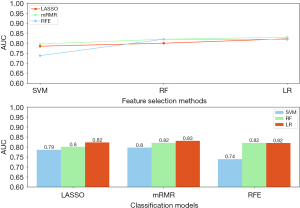
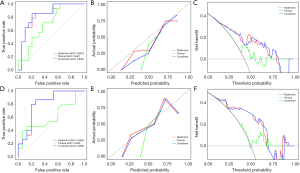
Table 2
| Model | AUC | Accuracy (%) | Sensitivity (%) | Specificity (%) | Precision (%) | F1-score (%) |
|---|---|---|---|---|---|---|
| Internal | ||||||
| Radiomics | 0.823 (0.674–0.973) | 75.8 (60.6–87.9) | 78.6 (53.8–100) | 73.7 (52.4–92.9) | 68.8 (43.8–91.7) | 73.3 (51.9–88.3) |
| Clinical | 0.697 (0.517–0.878) | 57.6 (39.3–72.7) | 71.4 (42.9–92.9) | 47.4 (25.0–70.0) | 50.0 (26.7–71.4) | 58.8 (34.5–76.1) |
| Combined | 0.838 (0.695–0.981) | 78.8 (63.6–90.9) | 85.7 (63.6–100) | 73.7 (52.3–91.7) | 70.6 (44.4–91.7) | 77.4 (57.1–90.9) |
| External | ||||||
| Radiomics | 0.804 (0.637–0.971) | 80.0 (66.7–93.3) | 86.7 (66.7–100) | 73.3 (50.0–93.3) | 76.5 (55.5–94.4) | 81.2 (64.0–94.1) |
| Clinical | 0.609 (0.396–0.822) | 50.0 (33.3–66.7) | 53.3 (26.7–78.6) | 46.7 (20.0–71.4) | 50.0 (26.7–78.6) | 51.6 (27.6–70.6) |
| Combined | 0.809 (0.645–0.973) | 80.0 (66.7–93.3) | 86.7 (66.7–100) | 73.3 (50.0–93.3) | 76.5 (55.5–94.4) | 81.2 (64.1–94.1) |
The 95% confidence intervals for each index are shown in parentheses. AUC, area under the curve.
The Delong test was employed to facilitate a comparative and analytical assessment of the clinical, radiomics, and combined models generated. As evidenced in Table S5, no notable discrepancy was observed between the radiomics model and the clinical-radiomics model in either the test case group of our hospital or the test case group of our branch hospital. Conversely, a notable divergence was evident when the clinical model was compared with the radiomics model or the combined model. The diagnostic performance improvements were statistically significant.
Model interpretation
The average SHAP values of the selected clinical characteristics and radiomics features in the training set were calculated based on the LR classification model. Bar plots, swarm plots, and heat maps were then generated using the Pyradiomics package in Python 3.9.13., as illustrated in Figure 6. Both clinical and imaging features contributed to the prediction of the model, but it was easy to note that radiomic features were more important than clinical and demographic features and dominated the integrated diagnostic model. In addition, we also supplemented the permutation importance analysis. The top five most important features identified by this analysis are basically consistent with those identified by SHAP (as shown in Figure 7).
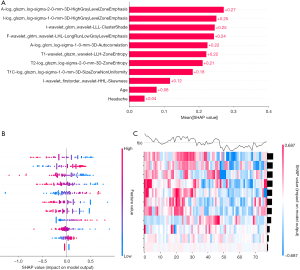
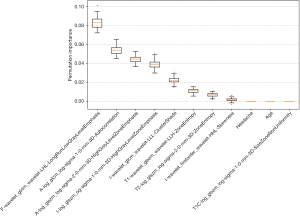
Discussion
Currently, the diagnostic methods for ICGCTs mainly include consistent diagnosis of laboratory and imaging examinations (8,22), diagnostic treatments, and pathological diagnosis. In practical work, invasive examinations are highly risky due to the nature of the tumour and its proximity to adjacent structures (23). From the perspective of imaging, safety is one of its greatest advantages. In previous studies, Wu et al. analysed the imaging manifestations of germinomas and NGGCTs on MRI, highlighting significant differences in tumour size, location, T1 high signal foci, cystic components, tumour edges, and enhancement patterns (24). Morana et al. and Lou et al. also proposed that the evaluation of susceptibility-weighted imaging (SWI) or gradient recalled echo (GRE) imaging features of ICGCTs may assist in distinguishing between different tumour types (25,26). However, quantifying these differences into a standardised format poses challenges, making it difficult to provide specific recommendations on the histology of ICGCTs based solely on imaging findings in practical work. In the field of radiomics, digitized encrypted medical images, which contain information related to tumour pathophysiology, are transformed into mineable high-dimensional data (27). These data can be comprehensively utilised through quantitative image analysis, improving a potential way to enhance the efficiency of imaging diagnosis. In recent years, within the realm of radiomics, research on diseases involving various organs and systems has been continuously advancing. Regarding germ cell tumours, Feliciani et al. previously employed radiomics methods to distinguish testicular germinomas from NGGCTs (28). Combining the germ cell theory proposed by Teilum (29,30) with the macroscopic, microscopic, and immunohistochemical similarities between intracranial germinomas, testicular seminomas, and ovarian dysgerminomas (31), it further demonstrates the potential of radiomics in the differential diagnosis of histological types of ICGCTs.
In this study, we constructed and tested ML models for differentiating germinomas from NGGCTs under multiple combinations. The results showed that the model with the best performance was the combination of LASSO and LR, with an AUC value of 0.823 in the test set. On further examination of the external test set, the AUC remained above 0.8. Besides, another clinical-radiomics model was constructed by combining the clinical features, with AUC values of internal 0.838 and external 0.809. Both models showed good diagnostic performance, robustness, and interpretability.
Previous radiomics studies have been conducted on ICGCTs. For instance, in the report by Ye et al. (32), they attempted to differentiate germinomas from other pineal region tumours (including NGGCTs) using ML based on clinical and radiomics. The features of the tumour and peritumoral oedema were extracted from T1WI, T2WI, T2-FLAIR, and contrast-enhanced T1WI sequences, and various combinations of these sequences were explored to determine the most effective combination for the optimal classification model. Ultimately, the random forest classification model, utilising the combination of oedema-tumour (region of interest, ROI) and T2-FLAIR (MRI sequence), performed the best among all the pure radiomics models, achieving an AUC of 0.80 [95% confidence interval (CI): 0.74–0.86]. Besides, Fan et al. conducted a study aiming to differentiate between germinomas and pineal parenchymal tumours using clinical radiomics. They extracted 1,562 radiomics features from T2WI and gadolinium-enhanced T1WI MRI images. Elastic net and SVM algorithms were employed for feature selection and classification algorithms, and the AUCs of the radiomics and clinical radiomics models reached 0.88 and 0.94, respectively (33).
Collectively, these studies were conducted in the context of marked heterogeneity in clinical management strategies for germinoma, with each investigation developing robust diagnostic models utilising radiomics methodologies. Building upon this foundational premise, our investigation distinguishes itself through dual emphasis on histopathological subtyping of neoplasms and comprehensive analysis of lesion distribution across anatomical compartments. Moreover, our MRI protocol systematically incorporated all the sequences requisite for diagnosis. SHAP-guided interpretability analysis revealed that the underlying biological information derived from diffusion-related imaging biomarkers held a predominant position in the diagnostic model’s feature importance hierarchy. And the analysis of permutation importance also supports this point.
In the radiomics features, it was noteworthy that following the application of feature selection, the retained features were all transformed features, with texture features representing the majority, including Gray-Level Co-Occurrence Matrix (GLCM), Gray-Level Size-Region Matrix (GLSZM), and Gray-Level Run-Length Matrix (GLRLM). The texture features reflect the degree or sudden change of intensity fluctuation of gray level on the image (34). GLCM provides a description of entropy, grayscale inhomogeneity, and other relevant information. GLSZM and GLRLM quantify the number of groups of interconnected neighbouring pixels or voxels with the same grey level. However, Gray-Level Distance Zone Matrix (GLDZM) not only assesses regions that are connected to adjacent pixels or voxels with the same grey level, but it also requires that they be situated at the same distance from the ROI edge (35). Furthermore, in the LoG filter, the transformed features with low and medium filter values (α=1, 2) were retained, suggesting that in the fine and medium texture, germinoma and NGGCTs are more likely to have significant differences. Previous studies have shown that texture analysis can effectively measure intratumor heterogeneity; for example, Skogen et al. found that coarse texture entropy and uniformity can distinguish low-grade from high-grade tumours (36).
Similar to most radiomics studies, demographics were also taken into consideration as potential factors. Based on this, a clinical model and a clinical - radiomics model were established. However, the clinical model showed poor diagnostic performance, with an AUC of less than 0.7. The DeLong test showed that the performance of the radiomics model and the clinical-radiomics model was significantly better than that of the clinical model, while there was no significant difference between the radiomics model and the clinical-radiomics model. After adjusting the P values using false discovery rate (FDR) correction in clinical statistical analyses, we observed that age and headache manifestations no longer retained statistical significance, and the moderate effect sizes calculated for these features may partially explain the suboptimal diagnostic performance. However, underpowered statistical analyses due to limited sample size, combined with the consistent patterns between the main hospital cohort and overall population (Tables S6,S7), suggest that these clinical factors should not be definitively excluded as non-informative clinical features.
Although we can partially diagnose NGGCTs with the help of tumour markers, as per the diagnostic guidelines, the majority of patients require biopsies for diagnostic purposes. It has been highlighted in several reports that even partial or complete removal of the tumour may be an inaccurate procedure, and it is estimated the error rate of cancer histopathology can be as high as 23% (37). Moreover, within the realm of ICGCTs, mixed germ cell tumours account for around 20% of the cases (38). This proportion significantly compounds the substantial uncertainty in the ultimate determination of histopathology biopsy. During the data collection stage, two patients who had initially been diagnosed with germinomas were subsequently diagnosed with NGGCTs upon further confirmation. In comparison to biopsy, imaging has the capacity to provide a more comprehensive display of the tumour as a whole. A number of studies have demonstrated a strong correlation between radiomics features and cellular-level heterogeneity indexes (39,40). In this study, radiomics models demonstrated excellent diagnostic performance, with good accuracy, confirming their potential value and feasibility.
There are several limitations in this study. First, regarding sample size considerations, although the diagnostic model demonstrated favorable performance and balanced classification efficacy (YI =0.6) in the test set, and several dominant features exhibited consistent statistical significance across both training and test cohorts, most features showed insufficient statistical power due to the limited sample size except for the feature of a-log_glszm_log-sigma-2-0 mm-3d-highgraylevelzone; as ICGCTs are rare central nervous system tumours (Table S8). Further research would benefit from the use of large-scale data from multiple centres in order to validate the effectiveness and generalizability of the models. Secondly, the study is retrospective and is therefore susceptible to the limitations inherent to such a design, including the potential for selection bias or information bias. In conclusion, although our study included the most common five MRI sequences, the addition of advanced MRI sequences, such as SWI, may provide further diagnostic information and enhance the overall diagnostic performance.
Conclusions
In summary, this study represents an initial attempt to utilise radiomics for the histological subtype discrimination of germ cell tumours involving different anatomical locations. Additionally, it evaluates feature contribution values through two distinct feature importance analysis methods and verifies their consistency. The results demonstrate that radiomics-based ML has considerable diagnostic value for the differential diagnosis of germinoma and NGGCTs. However, the value of clinical information for classification is relatively limited.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-210/rc
Data Sharing Statement: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-210/dss
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-210/prf
Funding: This study was supported by grants from
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-210/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments, and obtained approval from the Ethics Committee of the Children’s Hospital of Chongqing Medical University [(2023) No. 7]. Written informed consent was waived for this retrospective study by the Institutional Review Board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kuratsu J, Ushio Y. Epidemiological study of primary intracranial tumours: a regional survey in Kumamoto prefecture in the southern part of Japan. J Neurosurg 1996;84:946-50. [Crossref] [PubMed]
- Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 1985;63:155-67. [Crossref] [PubMed]
- Araki C, Matsumoto S. Statistical reevaluation of pinealoma and related tumors in Japan. J Neurosurg 1969;30:146-9. [Crossref] [PubMed]
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 2021;23:1231-51. [Crossref] [PubMed]
- Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist 2008;13:690-9. [Crossref] [PubMed]
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109. [Crossref] [PubMed]
- Frappaz D, Dhall G, Murray MJ, et al. EANO, SNO and Euracan consensus review on the current management and future development of intracranial germ cell tumors in adolescents and young adults. Neuro Oncol 2022;24:516-27. [Crossref] [PubMed]
- Qaddoumi I, Sane M, Li S, et al. Diagnostic utility and correlation of tumor markers in the serum and cerebrospinal fluid of children with intracranial germ cell tumors. Childs Nerv Syst 2012;28:1017-24. [Crossref] [PubMed]
- Hu M, Guan H, Lau CC, et al. An update on the clinical diagnostic value of β-hCG and αFP for intracranial germ cell tumours. Eur J Med Res 2016;21:10. [Crossref] [PubMed]
- Murray MJ, Bartels U, Nishikawa R, et al. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol 2015;16:e470-7. [Crossref] [PubMed]
- Handa H, Yamashita J. Current treatment of pineal tumors (author's transl). Neurol Med Chir (Tokyo) 1981;21:147-54. [Crossref] [PubMed]
- Qiu X, Luo S, Ma Z, et al. Preliminary Dosage Research on Diagnostic Radiation of Intracranial Germinoma. Journal of Capital University of Medical Sciences 2006:395-96.
- Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 2013;15:788-96. [Crossref] [PubMed]
- Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation--a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 1996;14:2908-15. [Crossref] [PubMed]
- Fetcko K, Dey M. Primary Central Nervous System Germ Cell Tumors: A Review and Update. Med Res Arch 2018;6:1719. [Crossref] [PubMed]
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016;278:563-77. [Crossref] [PubMed]
- Duron L, Sadones F, Thiesse P, et al. Loco-regional extensions of central nervous system germ cell tumors: a retrospective radiological analysis of 100 patients. Neuroradiology 2018;60:27-34. [Crossref] [PubMed]
- Yang QY, Guo CC, Deng ML, et al. Treatment of primary intracranial germ cell tumors: Single center experience with 42 clinically diagnosed cases. Oncotarget 2016;7:60665-75. [Crossref] [PubMed]
- Wu J, Xia Y, Wang X, et al. uRP: An integrated research platform for one-stop analysis of medical images. Front Radiol 2023;3:1153784. [Crossref] [PubMed]
- van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 2017;77:e104-7. [Crossref] [PubMed]
- R Core Team (2024). _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/
- Kanamori M, Kumabe T, Tominaga T. Is histological diagnosis necessary to start treatment for germ cell tumours in the pineal region? J Clin Neurosci 2008;15:978-87. [Crossref] [PubMed]
- Chiba K, Aihara Y, Komori T, et al. Placental alkaline phosphatase in cerebrospinal fluid as a biomarker for optimizing surgical treatment strategies for pineal region germ cell tumors. Brain Tumor Pathol 2020;37:60-8. [Crossref] [PubMed]
- Wu CC, Guo WY, Chang FC, et al. MRI features of pediatric intracranial germ cell tumor subtypes. J Neurooncol 2017;134:221-30. [Crossref] [PubMed]
- Morana G, Alves CA, Tortora D, et al. T2*-based MR imaging (gradient echo or susceptibility-weighted imaging) in midline and off-midline intracranial germ cell tumours: a pilot study. Neuroradiology 2018;60:89-99. [Crossref] [PubMed]
- Lou X, Ma L, Wang FL, et al. Susceptibility-weighted imaging in the diagnosis of early basal ganglia germinoma. AJNR Am J Neuroradiol 2009;30:1694-9. [Crossref] [PubMed]
- Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14:749-62. [Crossref] [PubMed]
- Feliciani G, Mellini L, Carnevale A, et al. The potential role of MR based radiomic biomarkers in the characterization of focal testicular lesions. Sci Rep 2021;11:3456. [Crossref] [PubMed]
- Teilum G. Endodermal sinus tumors of the ovary and testis. Comparative morphogenesis of the so-called mesoephroma ovarii (Schiller) and extraembryonic (yolk sac-allantoic) structures of the rat's placenta. Cancer 1959;12:1092-105. [Crossref] [PubMed]
- Teilum G. Classification of endodermal sinus tumour (mesoblatoma vitellinum) and so-called "embryonal carcinoma" of the ovary. Acta Pathol Microbiol Scand 1965;64:407-29. [Crossref] [PubMed]
- Bentley AJ, Parkinson MC, Harding BN, et al. A comparative morphological and immunohistochemical study of testicular seminomas and intracranial germinomas. Histopathology 1990;17:443-9. [Crossref] [PubMed]
- Ye N, Yang Q, Liu P, et al. A comprehensive machine-learning model applied to MRI to classify germinomas of the pineal region. Comput Biol Med 2023;152:106366. [Crossref] [PubMed]
- Fan Y, Huo X, Li X, et al. Non-invasive preoperative imaging differential diagnosis of pineal region tumor: A novel developed and validated multiparametric MRI-based clinicoradiomic model. Radiother Oncol 2022;167:277-84. [Crossref] [PubMed]
- Mayerhoefer ME, Materka A, Langs G, et al. Introduction to Radiomics. J Nucl Med 2020;61:488-95. [Crossref] [PubMed]
- Thibault G, Angulo J, Meyer F. Advanced statistical matrices for texture characterization: application to cell classification. IEEE Trans Biomed Eng 2014;61:630-7. [Crossref] [PubMed]
- Skogen K, Ganeshan B, Good T, et al. Imaging heterogeneity in gliomas using texture analysis. CancerImaging 2011;11:S113.
- Nguyen PL, Schultz D, Renshaw AA, et al. The impact of pathology review on treatment recommendations for patients with adenocarcinoma of the prostate. Urol Oncol 2004;22:295-9. [Crossref] [PubMed]
- Ironside JW, Moss TH, Louis DN, et al. Diagnostic Pathology of Nervous System Tumours. AJNR Am J Neuroradiol 2006;27:1385-7.
- Moon SH, Kim J, Joung JG, et al. Correlations between metabolic texture features, genetic heterogeneity, and mutation burden in patients with lung cancer. Eur J Nucl Med Mol Imaging 2019;46:446-54. [Crossref] [PubMed]
- Choi ER, Lee HY, Jeong JY, et al. Quantitative image variables reflect the intratumoral pathologic heterogeneity of lung adenocarcinoma. Oncotarget 2016;7:67302-13. [Crossref] [PubMed]


