
Case report: diagnostic challenges and long-term survival of a child with bronchial mucoepidermoid carcinoma
Highlight box
Key findings
• We report the first pediatric case of bronchial mucoepidermoid carcinoma (MEC) with CRTC3::MAML2 fusion gene, overcoming initial diagnostic challenges and achieving treatment successful at a tertiary pediatric hospital in Vietnam with support from international experts at St Jude Children’s Research Hospital.
What is known and what is new?
• Bronchial MEC is a rare primary pulmonary cancer in children and overall survival rate can reach 100% with complete surgical resection. International collaboration is crucial for supporting developing countries like Vietnam in achieving accurate diagnosis and effective treatment.
• The CRTC3::MAML2 fusion gene, detected via RNA sequencing, confirmed the definite diagnosis of bronchial MEC—a novel finding in this context.
What is the implication, and what should change now?
• Patients with histopathological findings inconsistent with clinical presentation, especially in rare cancer like bronchial MEC, should be referred to experienced international experts to accurate diagnosis and tailored treatment.
Introduction
Bronchial mucoepidermoid carcinoma (MEC) is a rare malignant neoplasm in children. The incidence of MEC has remained stable at 0.049 per 100,000 people and the median age at diagnosis is 16 years old (1). MEC accounts for 9% of patients with primary pulmonary cancer in children (2). The slow growing tumors can narrow and block the bronchi, causing pneumonia and lung collapse. Morphological characteristics of MEC include a variable number of mucus-secreting, epidermoid and intermediate cells (3,4). MEC is typically characterized by the t(11;19) (q21; p13) translocation; resulting in fusion of the MECT1 and MAML2 (5,6). However, diagnosis can be challenging, as the tumor may mimic primary non-small cell lung carcinoma (7). The 5-year overall survival for MEC in literature was 100% (2). Herein we report on the case of a 15-year-old diagnosed with bronchial MEC with CRTC3::MAML2 fusion gene. International consultation was key in providing the patient with an accurate diagnosis and appropriate treatment at the tertiary pediatric hospital in Vietnam. We present this article in accordance with the CARE reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-235/rc).
Case presentation
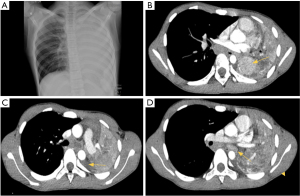
A 15-year-old male presented in December 2019 with an 8-month history of hemoptysis that worsened acutely, with fever, cough, left chest pain, and difficulty breathing for 5 days. Family history was positive for a family member with tuberculosis 2 years prior to this presentation. He was admitted to the Center for Tropical Diseases at Vietnam National Children’s Hospital (VNCH) due to respiratory failure and requiring oxygen via mask. Examination revealed dullness and decreased breath sounds in the left lung. C-reactive protein was 115 mg/L. Lung ultrasound revealed consolidation in the left lower lobe. Chest X-ray (Figure 1A) suggested complete left lung collapse. Chest computed tomography (CT) (Figure 1B-1D) confirmed total left lung collapse with a 46 mm × 26 mm enhancing solid mass near the left hilum, with ill-defined margins and no air bronchograms. A left pleural effusion (9 mm thick) was noted, predominantly in the posterior costophrenic angle. Additionally, multiple enlarged lymph nodes are seen below the aortic arch with the biggest one (10 mm × 25 mm × 22 mm).
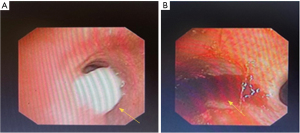
The patient was initially treated with antibiotic therapy, but his condition worsened. A flexible bronchoscopy in January 2020 revealed that a mass in the distal left main bronchus, partially blocking the main bronchial aperture. Because of active bleeding no biopsy was performed (Figure 2).
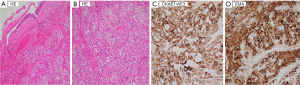
Multidisciplinary emergency consultation at VNCH including the infectious disease center, general surgical center, respiratory center, anesthesia department, surgical intensive care unit (ICU), and oncology center recommended open biopsy and control of severe hemoptysis in February 2020. During the first prompt surgery, due to hemodynamic instability and bleeding from the lower lobe, a left thoracotomy was performed for open biopsy, lower lobectomy, and without lymph node below aortic arch dissection. Pathology was initially consistent with the diagnosis of clear cell carcinoma either primary or metastasis to lungs. The morphological features were of malignant cells with wide, bright cytoplasm and large, irregular nuclei, low nuclear/cytoplasmic ratio, arranged into irregular acinar structures. Immunohistochemical staining confirmed the tumor’s bronchial mucosal origin with positivity for hematoxylin and eosin (HE) (+), epithelial membrane antigen (EMA) (+), cytokeratin (CK) AE1/AE3 (+), and negativity for HMB45, Melan A, smooth muscle actin (SMA), Desmin, CD1a (Figure 3). The specimen was reviewed at the tertiary oncology hospital in Vietnam, and the pathologist also considered the possibility of clear cell carcinoma. As no kidney mass was observed, and given the rarity of this case, the specimen was sent to the Pathology Department of St Jude Children’s Research Hospital (SJCRH), USA to consult.
On this second review, the morphological features were consistent with a malignant neoplasm composed of large tumor cells with abundantly clear cytoplasm and distinct cell boundaries, with moderate pleomorphism. Based on morphology alone, the differential diagnosis included metastatic renal cell carcinoma (RCC)—either the clear cell or translocation-associated type—alveolar soft part sarcoma, and clear cell lung tumor (part of the PEComa family of tumors). Immunohistochemistry (IHC) performed at SJRCH showed strong reactivity for CK AE1/AE3, CK Cam5.2, and EMA—as was seen in VNCH and ruled out the possibility of alveolar soft part sarcoma and the PEComa family of tumors. The tumor cells also showed strong nuclear reactivity for TFE3. CK 7 was positive, whereas CK 20, CD10, S100, TTF1, CDX2, p63, and Melan-A were all negative. Primary lung tumors are usually positive for the TTF1 transcription factor but in our case it was negative. The TFE3 fluorescence in situ hybridization (FISH) performed at Mayo Clinic showed negative for rearrangement. The RNA sequencing (RNA-seq) to be performed at SJRCH and showed a CRTC3::MAML2 fusion. This finding, together with morphology, would support the diagnosis of MEC.
After tumor board meeting with experts from SJRCH and reviewing articles, the possibility of complete resection was challenging, and the decision was made to attempt tumor reduction with neoadjuvant PLADO (cisplatin and doxorubicin) chemotherapy to facilitate surgery. Since MEC is a glandular-squamous carcinoma and there is no standardized chemotherapy regimen, protocols include platinum-based regimens and could add doxorubicin in case if unresectable, recurrent, or metastatic. The patient received two courses of cisplatin 80 mg/m2 continuous 24 hours intravenous (IV) and doxorubicin 60 mg/m2 continuous 48 hours IV in March and April 2020. The CT scan after chemotherapy showed the tumors had shrunk and were surrounding the left bronchus with a diameter of 27 mm × 29 mm × 33 mm. In the second surgery in May 2020, only about 70% of the tumor surrounding the left bronchus could be removed, as it was inseparable from the bronchus and vessels. The remaining of the tumor was encasing the left bronchus and vessels, but there were not lymph nodes (Figure 4).
Pathology after second surgery at VNCH confirmed bronchial MEC and tumor cells present at surgical margins. The patient showed no difficulty breathing and gained 2 kg. After the second surgery, the experts at SJCRH advised that a left pneumonectomy should be performed. The patient received one additional course of chemotherapy in June 2020 while waiting to recover in anticipation for a third surgery. A flexible bronchoscopy and endobronchial biopsy were performed before third surgery. The left main bronchus was normal. The lower lobe bronchus was truncated. The upper lobe bronchus was normal. There was no granulation tissue (Figure 5). The biopsy results revealed no tumor cells at the remnant of the left bronchus.
The patient underwent the third surgery with left pneumonectomy through a midline sternotomy and left posterolateral thoracotomy approach in July 2020. No lymph nodes were found during this surgery. Intraoperative pathology evaluation showed that there were no carcinoma cells at the proximal end of the left bronchus. The left bronchus was then closed with continuous running 5.0 polypropylene suture, the left pulmonary artery and left pulmonary vein were divided and closed with running suture 6.0 polypropylene. The patient was extubated after one day.
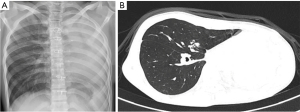
The child received a chest X-ray follow-up every 3 months within the first 3 years since the day of treatment completion and every 6 months in the following 2 years. Additionally, a following-up CT scan image showed no tumor relapse after one year of treatment completion (Figure 6). He has been free of disease with a normal respiratory function after 5-year follow-up. Currently, the patient is a university student and participates in light sports activities.
All procedures performed in this study were in accordance with the ethical standards of the Vietnam National Children’s Hospital research committee and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient and his family for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Primary malignant lung tumors of epithelial origin in pediatric patients are rare, with an average of 16 cases per year in the USA from 1998 to 2011 (1). The most common histological types include carcinoid tumor, MEC, squamous cell carcinoma (SCC), adenocarcinoma, bronchoalveolar carcinoma (BAC), and small cell carcinoma (SCLC). The tumor originates from the secretory ducts of the mucous-secreting glands beneath the respiratory mucosa. MEC accounts for 10–18% of primary malignant pulmonary tumors in children and is the second most common type (2). MEC of the bronchi is rare.
Although MEC can occur at any age, it is rarely seen in children, especially young children under 10 years old (8). Therefore, MEC is often not initially considered in the differential diagnosis of children presenting with lung collapse or recurrent pneumonia, leading to delays in diagnosis (8-17). Common clinical symptoms of MEC include cough, hemoptysis, recurrent pneumonia, wheezing, fever, chest pain, and finger clubbing. Tumor growth can obstruct peripheral airway flow, leading to dyspnea, wheezing, and coughing. Prolonged obstruction leads to pneumonia, lung collapse, and bronchiectasis. In our study, the patient’s initial symptoms were recurrent hemoptysis lasting for 8 months and had initially been diagnosed with pneumonia. Antibiotic treatment and symptomatic relief medication initially reduced the disease symptoms, leading to a misdiagnosis. Based on this case, we recommend that MEC be included in the differential diagnosis of patients presenting with recurrent hemoptysis, cough, and pneumonia-like symptoms.
Investigations include chest X-ray, bronchoscopy, and lung CT scan (4). Chest X-ray may show lobar pneumonia, atelectasis, pleural effusion, a hilar mass or nodule, present in 66% of bronchial MEC cases (18). Lung CT scan is useful for localizing and characterizing MEC in children, typically revealing well-defined, homogeneously enhancing solid lesions with rare calcifications. Colletti et al. found that a high-resolution CT scan had a sensitivity of up to 80% for detecting MEC tumors (19).
Surgical intervention for the tumor aims to obtain diagnostic tissue samples for histopathological examination and to excise the entire tumor while preserving as much healthy lung tissue as possible. However, in most cases, it may be necessary to remove the affected lobe or the entire lung (4,20). In our study, due to the tumor’s location, complete excision of the tumor was challenging, ultimately requiring a pneumonectomy.
In general, MEC tumors appear gray in color with well-defined borders and multiple polypoid masses, making them easily observable through bronchoscopy, where they appear as polypoid, exophytic or sessile tumors in major bronchi, often >5 cm. MEC are morphologically defined by the presence of variable proportions of epidermoid, mucous and intermediate cells in the absence of keratinization. Mucus secreting cells are usually large with light blue-gray mucinous cytoplasm; variants include columnar, goblet, cuboidal, clear or oncolytic cells. Immunohistochemistries are positive for CK7, CK AE1/AE3, p63, p40, mucicarmine and negative for TTF1, CK20 (10,21). Diagnostic cytology using sputum, bronchial washings, or bronchoalveolar lavage fluid is of limited value due to the tumor is often being covered by intact respiratory mucosal cells (5,6).
Bronchial MEC is characterized by the chromosomal translocation t(11;19) (q21;p13), which leads to fusion of the MECT1 and MAML2 genes (7,21,22). Analyzing the MAML2 gene mutation can help strengthen the diagnosis in challenging cases. Salem et al. found eight out of nine patients with pulmonary MEC had MECT1-MAML2 (6). The presence of MECT1-MAML2 was evaluated with reverse transcription polymerase chain reaction using RNA extracted from formalin-fixed paraffin-embedded tumor tissue (6).
In our study, based on histopathological characteristics of tumor cells and IHC, the initial diagnosis was a lung metastasis of a clear cell carcinoma. The morphology features of clear cell carcinoma and MEC are sometimes similar with large tumor cells with abundantly clear cytoplasm and IHC staining was positive for epithelial markers. Typical MEC cases may show mucinous epithelium, periodic acid schiff (PAS) (+), periodic acid schiff diastase (PASD) (+), mucicarmine and IHC staining positive with mucin 1 (MUC1), MUC2, MUC4 can help differentiate these two types of tumors. At that time, we had never encountered MEC before, so we chose clear cell carcinoma and did not make a differential diagnosis. However, a whole-body screening did not reveal a primary tumor at other sites. Further pathological evaluation at SJCRH showed overexpression of TFE3 by IHC, but without TFE3 rearrangement by FISH analysis. This confirmed the definitive diagnosis of bronchial MEC.
Prognostic factors for the disease include malignancy of the histopathological tissue, lymph node metastasis, size of tumor >5 cm and the ability to completely resect the tumor (6,9,20). Although tumors may invade through the bronchial wall, distant metastasis is rare. In children, MEC of the bronchial mucosal epithelium is considered malignant (9). These tumors grow slowly, and early diagnosis and definitive surgical treatment offer the best chance of cure for MEC patients (2,20). Most reported cases underwent radical surgery and did not experience recurrence (8-15). Our patient had a tumor of less than 5 cm, and lymph nodes below the aortic arch were gone after two courses of PLADO regimen. The tumor was completely resected with a margin free of tumor cells after the third surgery. Therefore, the patient survived over 5 years.
Conclusions
Bronchial MEC in children is rare, and clinical presentation is similar to that of other respiratory diseases, which can lead to a delay in early diagnosis. Identification of the CRTC3::MAML2 fusion gene can help confirm the diagnosis. The successful outcome in this case was made possible by the coordinated efforts of multiple specialties and close consultation with experts at SJCRH.
Acknowledgments
We would like to thank our colleagues at the Vietnam National Children’s Hospital for their dedicated care and contribution to the successful treatment of the patient.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-235/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-2025-235/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-2025-235/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Vietnam National Children’s Hospital research committee and with the Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the patient and his family for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Neville HL, Hogan AR, Zhuge Y, et al. Incidence and outcomes of malignant pediatric lung neoplasms. J Surg Res 2009;156:224-30. [Crossref] [PubMed]
- Rojas Y, Shi YX, Zhang W, et al. Primary malignant pulmonary tumors in children: a review of the national cancer data base. J Pediatr Surg 2015;50:1004-8. [Crossref] [PubMed]
- Fauroux B, Aynie V, Larroquet M, et al. Carcinoid and mucoepidermoid bronchial tumours in children. Eur J Pediatr 2005;164:748-52. [Crossref] [PubMed]
- Kalhor N, Moran CA. Pulmonary mucoepidermoid carcinoma: diagnosis and treatment. Expert Rev Respir Med 2018;12:249-55. [Crossref] [PubMed]
- Roden AC, García JJ, Wehrs RN, et al. Histopathologic, immunophenotypic and cytogenetic features of pulmonary mucoepidermoid carcinoma. Mod Pathol 2014;27:1479-88. [Crossref] [PubMed]
- Salem A, Bell D, Sepesi B, et al. Clinicopathologic and genetic features of primary bronchopulmonary mucoepidermoid carcinoma: the MD Anderson Cancer Center experience and comprehensive review of the literature. Virchows Arch 2017;470:619-26. [Crossref] [PubMed]
- Chan ES, Tong JH, To KF. CRTC3-MAML2 fusion transcript in a bronchial mucoepidermoid carcinoma. Pathology 2013;45:698-701. [Crossref] [PubMed]
- Qian X, Sun Z, Pan W, et al. Childhood bronchial mucoepidermoid tumors: A case report and literature review. Oncol Lett 2013;6:1409-12. [Crossref] [PubMed]
- Jaramillo S, Rojas Y, Slater BJ, et al. Childhood and adolescent tracheobronchial mucoepidermoid carcinoma (MEC): a case-series and review of the literature. Pediatr Surg Int 2016;32:417-24. [Crossref] [PubMed]
- Dinopoulos A, Lagona E, Stinios I, et al. Mucoepidermoid carcinoma of the bronchus. Pediatr Hematol Oncol 2000;17:401-8. [Crossref] [PubMed]
- Wildbrett P, Horras N, Lode H, et al. Mucoepidermoid carcinoma of the lung in a 6-year-old boy. Afr J Paediatr Surg 2012;9:159-62. [Crossref] [PubMed]
- Kitada M, Matsuda Y, Sato K, et al. Mucoepidermoid carcinoma of the lung: a case report. J Cardiothorac Surg 2011;6:132. [Crossref] [PubMed]
- Tri TT, Vu LT, My TT, et al. Mucoepidermoid lung carcinoma in a pediatric patient confused with pneumonia. Radiol Case Rep 2021;16:2749-53. [Crossref] [PubMed]
- Wordui SM, Lakhan A, Eze J, et al. Mucoepidermoid carcinoma of the bronchus in two children: Case reports. Respir Med Case Rep 2023;43:101858. [Crossref] [PubMed]
- Giusti RJ, Flores RM. Mucoepidermoid carcinoma of the bronchus presenting with a negative chest X-ray and normal pulmonary function in two teenagers: two case reports and review of the literature. Pediatr Pulmonol 2004;37:81-4. [Crossref] [PubMed]
- Omesh T, Gupta R, Saqi A, et al. A rare case of endobronchial mucoepidermoid carcinoma of the lung presenting as non-resolving pneumonia. Respir Med Case Rep 2018;25:154-7. [Crossref] [PubMed]
- Madafferi S, Catania VD, Accinni A, et al. Endobronchial tumor in children: Unusual finding in recurrent pneumonia, report of three cases. World J Clin Pediatr 2015;4:30-4. [Crossref] [PubMed]
- Voggel S, Abele M, Seitz C, et al. Primary lung carcinoma in children and adolescents - Clinical characteristics and outcome of 12 cases from the German registry for rare paediatric tumours (STEP). Lung Cancer 2021;160:66-72. [Crossref] [PubMed]
- Colletti PM, Beck S, Boswell WD Jr, et al. Computed tomography in endobronchial neoplasms. Comput Med Imaging Graph 1990;14:257-62. [Crossref] [PubMed]
- Chen W, Bai J, Fang Y, et al. Prognostic factors and surgical management in pediatric primary lung cancer: a retrospective cohort study using SEER data. Transl Pediatr 2024;13:1671-83. [Crossref] [PubMed]
- Huo Z, Wu H, Li J, et al. Primary Pulmonary Mucoepidermoid Carcinoma: Histopathological and Moleculargenetic Studies of 26 Cases. PLoS One 2015;10:e0143169. [Crossref] [PubMed]
- Zhang X, Bai QM, Yao QL, et al. MAML2 gene rearrangement, fusion patterns and clinicopathological characteristics in primary pulmonary mucoepidermoid carcinoma. Zhonghua Bing Li Xue Za Zhi 2021;50:891-8. [Crossref] [PubMed]




