Pharmacological treatment of anxiety disorders in children and adolescents: a review for practitioners
Introduction
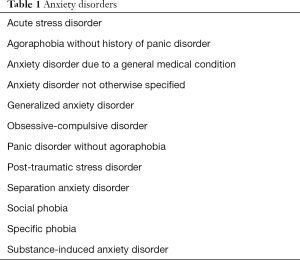
The key feature of anxiety is fear or apprehension by the child or adolescent of a future event (1-4). Feelings of anxiety are typically accompanied by emotional stress or tension, and somatic signs and symptoms (1-4). The main feature of an anxiety disorder is functional impairment from persistent fear or worry (1-6). Such impairment may affect functioning in school, play, work or interpersonal relationships (1-6). The symptoms of an anxiety disorder take up a significant amount of daily time of the child or the adolescent and such symptoms last over a period of several months, typically six or more (1). Different types of anxiety disorders are recognized based on their clinical presentation, acuity, and type of stimuli associated with anxiety (Table 1) (1).
Full table
Epidemiology
Anxiety disorders are common in children and adolescents with a reported prevalence between 10% and 30%, and a higher prevalence in females (1,3,5). A combination of genetic factors, temperamental characteristics of the child, and environmental risk factors play a role in the development of anxiety disorders (2,3,6). Although the age of onset varies depending on the specific disorder, most anxiety disorders are first recognized in late childhood to early adolescent years (1-4). While long term outcomes of childhood anxiety disorders have not been clearly elucidated, it is generally recognized that most tend to persist into adulthood (1-4). The long term impact of anxiety disorders on the psychosocial development of the child or adolescent is significant (1-4). Anxiety disorders also have a high rate of other comorbid mental health disorders (see Table 2) (1).

Full table
Clinical features
Normal developmental fears vary depending up on the age and developmental stage of the child and these should not be confused with anxiety disorders (4-6). Infants may be fearful of sudden loud noises and strangers; the preschool child may have fear of animals, the dark, imaginary creatures or the storms (3,6). Anticipatory anxiety may first manifest during preschool age (6). The school age child may have fears related to attending school that involve acceptance by peers at school or concern about a particular subject or even a specific teacher (2,3,6). During the adolescent years, fears may be regarding the future events, about one’s physical appearance, and sexuality (1-3). Conditions that should be considered in the differential diagnoses of anxiety disorders are listed in Table 3 (1-4).

Full table
Approach to treatment
In children and adolescents, a multimodal treatment approach is found to be the most effective approach. The multimodal approach comprises exposure-based cognitive behavior therapy, family therapy, patient and family education and the use of medications (3,5,7-22).
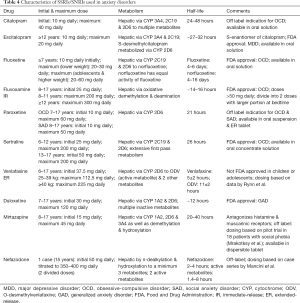
Selective serotonin reuptake inhibitors (SSRIs) have been shown to be effective in children and adolescents; whereas, the safety and efficacy of other drugs in the treatment of anxiety disorders in children and adolescents is not fully established (2,3,5,7-22). Our understanding of the safety, efficacy, and use of specific treatment modalities is informed by several key clinical trials: the Treatment of Adolescents with Depression Study (TADS) (23-30), the Child/Adolescent Anxiety Multimodal Study (CAMS) (31,32), the Research Unit on Pediatric Psychopharmacology (RUPP) Anxiety Study Group (33), the Pediatric Obsessive-Compulsive Disorder Treatment Study (POTS) (34), and the Treatment of Resistant Depression in Adolescents (TORDIA) (35-37). Our focus in this clinical review is on the use of medications in pediatric anxiety disorders, the key characteristics of which are summarized in Tables 4, 5 and 6 (16,38-45).
Full table

Full table

Full table
SSRIs
SSRIs are the recommended drugs of choice for pediatric anxiety disorders and found to be effective alone or in combination with cognitive behavior therapy (5,9,12,18). SSRIs act by blocking the reuptake of serotonin into pre-synaptic neurons and enhance serotonergic neurotransmission (6,14,18-20,46,47).
Side effects
Gastrointestinal side effects
Gastrointestinal side effects are common with SSRI use. Nausea, vomiting, diarrhea, flatulence, decreased appetite, dry mouth and heartburn are the most commonly reported side effects (12,15,18,20). Most are transient and resolve over a period of few weeks. In a few children and adolescents, weight gain may be a problem rather than weight loss and may not respond well to decreased caloric intake and increased physical activity (8,14,16,18).
Behavioral activation
Behavioral activation is much more common in children and adolescents than in adults and reported incidence ranges from 20% to 50% (3,13,20,21,48-52). Activation is more common during the initial days and weeks of starting the SSRI and after an increase in dose (18,20). Behavioral activation is characterized by alteration in mood, dysphoria, altered cognition, nervousness, agitation, irritability and in severe cases by akathisia (uncomfortable sensation of restlessness that can be physical or psychological or both) (12,18-21). Although hypomania, mania, and acute psychotic reactions have also been reported, behavioral activation is neither an indication of nor predictive of bipolar disorder (3,6,20). Many symptoms of behavioral activation and discontinuation syndrome are similar. A careful documentation of adherence to taking SSRIs will favor the diagnosis of behavioral activation (3,8,11).
Switching (or bipolar switching)
In switching, the patient’s mood state changes from depressed or anxious mood to that of manic or hypomanic state. This clinical feature differentiates bipolar switching from behavioral activation. In behavioral activation the mood state does not change (19,20,46,52).
The patient and the family recognize these as new symptoms not present before the treatment was started with an SSRI agent (3). Switching is a less common but significant side effect of SSRIs in children and adolescents. Bipolar switching should be differentiated from behavioral activation. Symptoms of switching tend to occur later in the course of the treatment and may not abate even after discontinuation of the SSRI agent (4,6,20). Development of symptoms suggestive of bipolar disorder requires discontinuation of SSRIs and starting appropriate treatment for bipolar disorder after further evaluation (3,5,13,22). Children and adolescents when effectively treated with an SSRI agent for anxiety or depression may also then manifest symptoms of comorbid mental health disorders more clearly (e.g., symptoms of attention deficit hyperactivity disorder or conduct disorder) and require further evaluation and treatment for the specific comorbid disorder.
Serotonin syndrome (SS)
Although infrequent, SS is a serious treatment emergent adverse event associated with the use of SSRIs (20,46,50,52,53). SS is caused by excessive serotonergic neuronal activation. The likelihood of SS increases when the dose of a SSRI is high and when a patient is also taking multiple drugs with serotonergic activity (3,9,15,18,20). The clinical features of SS include agitation, confusion, tachycardia, hypertension, tremors, incoordination, muscular rigidity, myoclonus, hyperreflexia, fever, shivering, excessive sweating, diaphoresis, and diarrhea (20). The complications of SS include seizures, metabolic acidosis, rhabdomyolysis, disseminated intravascular coagulation, renal failure, respiratory failure, coma, and death (20,42). As soon as any clinical symptoms or signs of SS is recognized, all serotonergic drugs must be immediately discontinued, and appropriate emergent general medical care must be initiated (3,11,17-19).
Withdrawal or discontinuation syndrome
The symptoms of SSRI drug withdrawal may be seen in some children and adolescents when the SSRI is abruptly stopped following a period of regular use (3,6,7,9,18). It is more likely to occur with the use of shorter half-life SSRIs, longer duration of use and abrupt discontinuation (8,10,18,50). Withdrawal symptoms include gastrointestinal disturbances, dysphoric mood, irritability, headache, sweating, chills, fatigue, agitation, dizziness, sensory disturbances (e.g., electric shock like sensations), anxiety, confusion, and sleep disturbances (13,20,50,54,55). For most SSRIs, symptoms are seen within 2–5 days of reducing the dose or discontinuation of the medication; in the case of fluoxetine, because of its longer half-life, symptoms of discontinuation may not be seen until after 7–10 days of stopping the medication (14,16,18,20,46,50). Most symptoms associated with discontinuation of SSRI agents generally resolve within 1–2 weeks (20). Sometimes severe symptoms associated with discontinuation of an SSRI agent may require restarting the patient on an SSRI at a lower dose for a short period and gradually tapering the medication (2,11,20). Symptoms of discontinuation syndrome should be differentiated from those of anxiety disorder recurrence or relapse.
Suicide ideation
The potential risk for suicidality associated with the use of antidepressants continues to be a subject of much debate. In 2004, the U.S. Federal Drug Administration (FDA) conducted a pooled analysis of placebo-controlled trials in children and adolescent with major depressive disorder, obsessive compulsive disorder or other systematic psychiatric disorders. The analyses comprised of a total of 24 short-term trials of 9 antidepressant drugs (including SSRIs) in over 4,400 patients. Based on the findings of the analysis, the FDA issued a warning for all antidepressants regarding increased risk of suicidality among children and adolescents being treated with antidepressants. The FDA recommended that children and adolescents placed on antidepressants be monitored closely, especially during the first few days to week for any signs of increased suicidality. Multiple subsequent studies have not been able to conclusively show that antidepressant use in children and adolescents is associated with increased risk for suicidality (2,3,11-15,17,18,28,31).
Cardiovascular side effects
Cardiovascular side effects associated with SSRIs are uncommon; however, prolonged QT syndrome has been reported with higher doses and in cases of SSRI overdose (5,6,8,12,14,20). In practice, a routine electrocardiogram is not recommended before starting SSRI agent, unless otherwise indicated based on history and physical examination findings.
Sexual side effects
Some individuals report delayed orgasm, decreased sexual desire, and ineffective orgasm while taking SSRIs (46-50). Most of such sexual side effects decrease in frequency or resolve over time with continued use of SSRIs (46-50). SSRIs have been used to treat premature ejaculation in some cases because it delays orgasm.
Sleep disturbance
Disturbance in sleep pattern is a common side effect of SSRIs. Such sleep disturbances include delayed onset of sleep, frequent waking up, shortened duration of sleep, and abnormal dream states (8,10,12,14,50). Because of poor quality of sleep, children and adolescents often may experience daytime drowsiness. Administration of the medication in the morning, improved sleep hygiene or use of melatonin should be considered if sleep disturbance is significant.
Other side effects
Other less frequently reported side effects with the use of SSRIs include increased yawning, increased sweating, mammoplasia, gynecomastia, and bleeding tendencies (11-15,18). Some children and adolescents may experience significant increased sweating. Nighttime sweating may be severe enough to drench the bed sheets. A different SSRI agent may be tried in these cases; also terazosin has been shown to be effective in the treatment of increased sweating in such cases (18,19).
Mammoplasia in girls and gynecomastia in boys have also been reported (19). SSRI induced increase in prolactin can cause galactorrhea in both men and women. It may take several months to resolve following discontinuation of the SSRI agent.
Apathy or amotivational syndrome, syndrome of inappropriate antidiuretic hormone secretion, and increased bleeding tendencies including gastrointestinal bleeding have been reported in children and adolescents on SSRIs (18,20). Increased bleeding is due to functional impairment of platelet aggregation and requires discontinuation of the SSRI (18,20).
Precautions
There is an increased risk of adverse events with the use of SSRIs in children and adolescents who are significantly underweight, have underlying hepatic or renal disease, have a history of atrial tachycardia or conduction disorders, and those who have a history of excessive daytime sleepiness (18,20). SSRIs should not be used concomitantly with monoamine oxidase inhibitors (MAOIs) and should be used with extreme caution if other serotonergic agents are also being used. Since SSRIs are metabolized by cytochrome P450, interactions with concurrent medications should be assessed prior to initiation (18,20).
Use in practice
Behavioral treatment modalities are the mainstay of treatment of anxiety disorders in children and adolescents, which requires effective participation by the patient and family in behavioral treatment. However, for many children and adolescents the symptoms of anxiety are severe enough to preclude effective participation in behavioral treatment. In these children and adolescents, SSRIs are indicated in order to first ameliorate anxiety symptoms to a sufficient level to allow for effective participation in behavioral treatment (3). Also, when symptoms of anxiety are severe, SSRIs should be continued along with behavioral treatment.
Comorbid disorders must be recognized and appropriately treated at the same time. The response to pharmacotherapy varies depending upon the severity and specific type of the anxiety disorder and the efficacy ranges from 50% to more than 70% (2,3,5,22).
SSRIs are the drugs of choice for the treatment for treatment of anxiety disorders in children and adolescents (2,5,46-50). Numerous studies provide evidence for the safety and effectiveness of SSRIs for the treatment of anxiety disorders in children and adolescents (23-37). Studies suggest that treatment of anxiety disorders may require dosages that are relatively higher than those used in the treatment of depression and the response may take longer (2,16,20). There is no conclusive evidence that suggests superiority of one SSRI over another, and no long term studies are available to guide the decision for duration of treatment (8,54,55). SSRIs should be continued for initial period of at least 1 year. If the patient is stable for a year, SSRI agent may be discontinued and patient monitored for re-emergence of anxiety symptoms (2-5,9,14,20). If symptoms of anxiety recur, the SSRI should be re-started. (2-5,9,14,20).
Once started, the SSRI agent should be continued for a period of 4 weeks before considering an increase in the dose or changing to a different SSRI agent (4,20,49,50). Subsequent increase in SSRI dose should not occur less than every 4 weeks (5,8,18,19). The dose is titrated higher until symptoms of anxiety resolve, intolerable side effects emerge, or a maximum recommended dose is reached without improvement in symptoms (3,5,8).
Due to higher rates of drug metabolism in children and adolescents, dosing of certain medications that are typically given once daily in adults may have to be divided into twice daily dosing to prevent withdrawal effects (2,3,6,20).
In children and adolescents on SSRIs, no specific laboratory monitoring is indicated (8,18,19,50). Children and adolescents taking SSRIs should be monitored clinically for an increased risk for suicidal behaviors. According to the U.S. FDA, a patient taking SSRIs should be clinically followed every week for the first month of treatment, every 2 weeks during the second month of treatment, and at the end of the third month of treatment. Because multiple factors play a role in the effective treatment and emergence of side effects, most medical practitioners individualize their approach to such clinical follow up.
Serotonin norepinephrine reuptake inhibitors
Venlafaxine and duloxetine
Multiple studies have shown serotonin and norepinephrine reuptake inhibitors (SNRIs), venlafaxine and duloxetine, to be effective in anxiety disorders in children and adolescents (12-15,18,35). SNRIs block both the serotonin and norepinephrine reuptake and weakly inhibit dopamine reuptake leading to increased serotonergic, noradrenergic, and dopaminergic neurotransmission (6,18,19,20). Venlafaxine acts like an SSRI at low doses and provides dual mechanism at higher doses (1,18,20).
Side effects
Side effects of SNRIs are similar to those of SSRIs but also includes an increase in systemic blood pressure, especially dose-dependent increase in supine systolic and diastolic blood pressure has been reported (9,15,18,19). Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion are uncommon but have been reported with SSRIs and SNRIs (5,20). Nervousness, somnolence, nausea, decreased appetite, weight loss, constipation, increased sweating, dry mouth, dizziness, difficulty sleeping and sexual dysfunction are the most commonly reported side effects of venlafaxine and duloxetine (12,15,16,18,20).
Precautions
SNRIs have similar precautions and contraindications as those for SSRIs including risk for SS (especially when combined with other serotonergic drugs) and a warning for increased suicidality. If there is a history of hypertension, it should be controlled prior to initiating an SNRI. Recent cardiovascular or cerebrovascular events may preclude use of SNRIs, and these agents should be used with great caution in children and adolescents with a history of seizures (19). Venlafaxine dose should be reduced in significant renal impairment while duloxetine should be avoided.
Use in practice
Venlafaxine and duloxetine are not considered the initial drugs of choice for the treatment of anxiety disorders in children and adolescents (2,5). Duloxetine is approved by the U.S. FDA for use in the treatment of generalized anxiety disorder in the age group from 7 to 17 years. Venlafaxine is not FDA approved for use in children and adolescents; however, it has been used when a SSRI is found to be ineffective. Extended release venlafaxine has been reported to be effective for generalized anxiety disorder and social phobia (5,7,18).
In addition to the monitoring guidelines noted for SSRIs, blood pressure should be routinely monitored in children and adolescents on SNRIs while a fasting lipid profile should be checked periodically (3,7,18,19). The initial starting dose of venlafaxine ER is 37.5 mg daily and maximum dose varies based on weight (see Table 4). The starting dose of duloxetine is 30 mg daily and maximum dose is 120 mg daily in children and adolescents (4,19).
Mirtazapine and nefazodone
Rarely, mirtazapine and nefazodone may be considered for the treatment of pediatric anxiety disorders when first line drugs of choice have been ineffective. These drugs are not approved by U.S. FDA for anxiety disorders, and data and clinical experience are limited to provide meaningful guidance for their use in children and adolescents.
Mechanism of action
Nefazodone is a SARI (serotonin antagonist/reuptake inhibitor) antidepressant and works via antagonism at the post-synaptic serotonin 5-HT2 receptor; it also blocks pre-synaptic serotonin and norepinephrine reuptake (4,9,19,20). Mirtazapine is an antagonist of the pre-synaptic norepinephrine alpha-2 receptor contributing to a release of norepinephrine and serotonin. It also blocks post-synaptic 5-HT2 and 5-HT3 receptors (19).
Side-effects
Mirtazapine is quite sedating (especially at lower doses), frequently causes weight gain, may elevate serum cholesterol, triglycerides and transaminases (9,18,20). Other side effects of mirtazapine include dry mouth, constipation, dizziness, abnormal dreams, increased appetite, nausea, weakness, abdominal pain, and flu-like symptoms (9,18-20). A potentially serious side effect of nefazodone is unpredictable acute hepatic failure. Other side effects of nefazodone include nausea, dry mouth, constipation, dyspepsia, increased appetite and weight gain, headaches, dizziness, insomnia, agitation, confusion, blurred vision, decreased libido and postural hypotension (9,18-20).
Use in practice
Although no specific laboratory tests are recommended for mirtazapine and nefazodone use, in practice, it is prudent to periodically check a fasting lipid profile, complete blood count, liver function tests and closely monitor weight gain, body mass index, and blood glucose (3,4,19,20). In adults the initial dose for mirtazapine is 7.5–15 mg per day with target dose of 15–45 mg given before bedtime. The initial dose for nefazodone is 100 mg twice daily day with usual dosage range of 150–600 mg daily divided into 2 doses (4).
Buspirone
Mechanism of action
Buspirone is an azapirone with a high affinity for 5-HT1A and 5-HT2 serotonin receptors. Buspirone is a serotonin 1A partial agonist. The effectiveness of buspirone in ameliorating the symptoms of anxiety is attributed to its post-synaptic partial agonist actions (19-21,56).
Buspirone is rapidly absorbed after oral ingestion. Peak plasma level is reached between 40 and 90 minutes after oral dose and has an average elimination half-life of 2 to 3 hours (21,56).
Side effects
Side effects of buspirone are generally mild and include headache, dizziness, nervousness, drowsiness, nausea, weakness, blurred vision, and excitement (56). Buspirone use has not been associated with physical or psychological dependence and no significant withdrawal symptoms occur when stopped (20,21,56).
Precautions
Buspirone should not be used with MAOIs; certain SSRIs used concomitantly with buspirone may reduce its clearance and raise its plasma levels (20,21).
Use in practice
No serious side-effects have been reported with the use of buspirone in children and adolescents. Therefore, buspirone is sometimes used as an initial drug for the treatment of anxiety disorders in children and adolescents. It has also been used as an adjunctive agent along with SSRI (21,56). No specific laboratory monitoring is indicated with the use of buspirone. Buspirone is not FDA approved in children, but studies in the package insert indicate a starting dose of 5 mg twice daily and range of 10–30 mg daily divided in 2 doses (21,56). It can take up to 3 weeks for its optimal effects and improvement in the psychic symptoms precedes improvement in somatic symptoms of anxiety.
Propranolol
Propranolol reduces the neuronal sympathetic outflow by blocking the beta-adrenergic receptors (19). Beta-blockers differ in their degree of selectivity for beta 1 (cardiac) and beta 2 (non-cardiac) receptors (57). Beta-blockers also have different degrees of lipophilicity (57). The exact mechanism of action of beta-blockers on the central nervous system had not been clearly elucidated (4,19,20). Propranolol is non-selective beta-adrenergic receptor blocker and reduces peripheral autonomic tone, which is believed to result in amelioration of somatic symptoms of anxiety, its effect on reducing emotional symptoms of anxiety is not well established (4,19,20).
Side effects
Dizziness, fatigue, bradycardia, hypotension, gastrointestinal upset, and rashes are commonly reported side effects of propranolol (19,57,58). Bronchospasm and heart failure, although less frequent, are more serious side effects of propranolol (57,58). In some children and adolescents, propranolol use has been associated with the emergence of Raynaud phenomenon (57,58).
Precautions
The beta blockers are contraindicated in children and adolescents with sinus bradycardia and greater than first degree heart block, uncompensated heart failure, asthma, and sick sinus syndrome (57,58). Beta-blockers should be used with caution in patients with significant peripheral arterial disease, pheochromocytoma, diabetes mellitus, hyperthyroidism, myasthenia gravis and compensated heart failure due to risk for disease exacerbation (57). Propranolol can exacerbate depression in children and adolescents (3,20,57).
Use in practice
Evidence is insufficient to support the use of propranolol in the treatment of anxiety disorders in children and adolescents. Propranolol has been found to be effective when used in certain specific circumstances that provoke anxiety, such as performance anxiety or in athletes to ameliorate anxiety associated with competition (3-5). The initial dose is 10–20 mg daily with a target of 20–40 mg daily divided twice (58).
Because propranolol lowers systemic blood pressure and heart rate, both should be monitored periodically (57,58). Before initiating propranolol, a baseline electrocardiogram is recommended to detect asymptomatic cardiac conduction abnormalities (57,58). It is also recommended to obtain a fasting blood glucose level prior to initiating treatment with propranolol (57,58). Propranolol can sometimes cause elevations in serum potassium, aspartate aminotransferase, alanine aminotransferase, triglycerides and alkaline phosphatase as well as reduce high-density-lipoprotein (HDL) cholesterol (57,58). Because of the potential for a rebound increase in systemic blood pressure, propranolol should be gradually tapered over a 2-week period when no longer indicated (57,58).
Hydroxyzine
Mechanism of action
Hydroxyzine is an antihistamine and it acts by blocking the histamine-1 receptors. It also antagonizes the muscarinic receptors and 5-HT2a receptors which is thought to mediate is anxiolytic properties (59,60).
Side effects
Hydroxyzine may cause dry mouth, sedation, tremor, increased appetite, fatigue, dizziness and constipation (59,60).
Use in practice
Hydroxyzine is not recommended for use in the treatment of anxiety disorders in children and adolescents. The use of hydroxyzine is limited to special circumstances in children and adolescents with symptoms of anxiety associated with organic diseases, and medical procedures (59,60). It is sometimes used in allergic conditions associated with pruritus and anxiety, acute hysteria, and anxiety associated with alcohol withdrawal (59,60). See Table 4 for dosing guidelines.
Benzodiazepines (BDZs)
BDZs bind to the benzodiazepine receptors in the central nervous system at the gamma aminobutyric acid-A (GABA-A) ligand-gated chloride channel complex and enhance the inhibitory effects of GABA (19,21,57). BDZs facilitate the chloride conductance through GABA-regulated channels. Their therapeutic effects in reducing anxiety symptoms are believed to be due to inhibition of amygdala-centered neuronal circuits (59,60).
Side effects
Side effects of BZDs include sedation, cognitive blunting, dizziness, ataxia, nystagmus, depression, transitory hallucinations, memory impairment (typically anterograde amnesia), constipation, diplopia, hypotension, urinary incontinence or retention, fatigue, slurred speech, paradoxical hyperexcitability, and nervousness (19,21,47,59,60). Paradoxical reactions are a significant concern in children and adolescents. Such episodes are characterized by behavioral disinhibition, loss of control, increased anxiety, increased aggressiveness, rage reaction, nightmares and hallucinations.
Respiratory depression, liver toxicity, and blood dyscrasias are less frequent but serious adverse reactions associated with the use of BDZs (21,47,59,60). Drug accumulation can occur in those with hepatic or renal impairment (21,59,60).
Precautions
The risk for abuse and dependence is increased with higher dosages and long-term use of BDZs. They are classified as class IV controlled substances within the U.S. Tolerance can be seen with the hypnotic and sedative effects but not the anxiolytic properties of BZDs. To prevent withdrawal or rebound symptoms, BDZs should be tapered when discontinued.
BZDs are contraindicated in children and adolescents with narrow angle glaucoma (21,60). Caution should be exercised in those with a history of depression or respiratory disorders.
BDZs should not be used in adolescents with potential for drug abuse or drug dependence. When individuals are on BDZs for long-term, periodic complete blood count and liver function tests are indicated (2,4,19,21,47,59,60).
Use in practice
The evidence is insufficient to support the use of BDZs in the treatment of anxiety disorders in children and adolescents (59,60). Rarely, BDZs are used for short-term treatment to control of severe anxiety (4,19,21,59,60). BZDs are more commonly used for procedure related anxiety. For adults, clonazepam is approved by the U.S. FDA for panic disorder, lorazepam for generalized anxiety disorder, and alprazolam for panic disorder as well as generalized anxiety disorder (2,19,21,59,60). Diazepam, chlordiazepoxide, and oxazepam have FDA-approved dosing for generalized anxiety disorder in children and adolescents (see Table 4 for details).
Conclusions
Children and adolescents with symptoms of anxiety or anxiety disorders commonly present in the primary care setting. Signs and symptoms of anxiety include cognitive, physiological, and behavioral manifestations. Other mental health conditions that can be co-morbidities with anxiety disorders should be recognized. Behavioral treatment, especially various cognitive behavioral therapy approaches, has been shown to be efficacious in the treatment of anxiety disorders in children and adolescents. Some children and adolescents may need pharmacotherapy at the same time behavioral treatment is initiated to allow for more effective participation in the therapy. Generally, a multimodal approach that includes behavioral and pharmacotherapy has been shown to be the most effective. SSRIs are the drugs of choice in treating anxiety in children and adolescents. Data are insufficient to recommend any other class of anti-anxiety drugs in children for long term treatment. Most children and adolescents respond well to treatment with long lasting resolution of symptoms. Recurrence of the same, or development of a different type of anxiety disorder, is not uncommon and in most individuals, anxiety disorders tend to persist into adulthood requiring long-term treatment planning.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th edition. Washington DC: American Psychiatric Press, 2013:189-234.
- Boydston L, French WP, Varley CK. Anxiety disorders. In: Greydanus DE, Patel DR, Pratt HD, et al. editors. Behavioral Pediatrics, 4th edition. New York: Nova Biomedical, 2015:265-76.
- Patel DR, Brown KA, Greydanus DE. Anxiety disorders in children and adolescents. In: Greydanus DE, Calles L Jr, Patel DR, et al. editors. Clinical Aspects of Psychopharmacology in Childhood and Adolescence. New York: NOVA Science, 2017:117-28.
- Greydanus DE, Calles JL Jr, Patel DR. Pediatric and adolescent psychopharmacology. Cambridge, UK: Cambridge Univ Press, 2008:61-76.
- Connolly SD, Bernstein GA. Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry 2007;46:267-83. [Crossref] [PubMed]
- Freeman JB, Garcia AM, Leonard HL. Anxiety disorders. In: Lewis ML. editor. Child and adolescent psychiatry: A comprehensive textbook, 3rd editon. Philadelphia: Lippincott Williams Wilkins, 2002:821-33.
- Rapp A, Dodds A, Walkup JT, et al. Treatment of pediatric anxiety disorders. Ann N Y Acad Sci 2013;1304:52-61. [Crossref] [PubMed]
- Anxiety disorders in children and adolescents. In: Paton C, Taylor D, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. West Sussex: John Wiley & Sons, Ltd., 2015:369-73.
- Henry A, Kisicki MD, Varley C. Efficacy and safety of antidepressant drug treatment in children and adolescents. Mol Psychiatry 2012;17:1186-93. [Crossref] [PubMed]
- Correll CU, Kratochvil CJ, March JS. Developments in pediatric psychopharmacology: focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry 2011;72:655-70. [Crossref] [PubMed]
- Wehry AM, Beesdo-Baum K, Hennelly MM, et al. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep 2015;17:52. [Crossref] [PubMed]
- Strawn JR, Welge JA, Wehry AM, et al. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety 2015;32:149-57. [Crossref] [PubMed]
- Creswell C, Waite P. Recent developments in the treatment of anxiety disorders in children and adolescents. Evid Based Ment Health 2016;19:65-8. [Crossref] [PubMed]
- Hussain FS, Dobson ET, Strawn JR. Pharmacologic treatment of pediatric anxiety disorders. Curr Treat Options Psychiatry 2016;3:151-60. [Crossref] [PubMed]
- Dobson ET, Strawn JR. Pharmacotherapy for pediatric generalized anxiety disorder: a systematic evaluation of efficacy, safety and tolerability. Paediatr Drugs 2016;18:45-53. [Crossref] [PubMed]
- Strawn JR, Sakolsky DJ, Rynn MA. Psychopharmacologic treatment of children and adolescents with anxiety disorders. Child Adolesc Psychiatr Clin N Am 2012;21:527-39. [Crossref] [PubMed]
- Fisher PH, Tobkes JL, Kotcher L, et al. Psychosocial and pharmacological treatment for pediatric anxiety disorders. Expert Rev Neurother 2006;6:1707-19. [Crossref] [PubMed]
- Weston C. Antidepressant drugs. In: Klykylo WM, Bowers R, Weston C, et al. editors. Green’s child and adolescent clinical psychopharmacology. Philadelphia: Lippincott Williams and Wilkins, 2014:186-257.
- Stahl SM. Essential Psychopharmacology: The Prescriber’s Guide. Cambridge, UK: Cambridge Univ Press, 2006.
- Chiu S, Leonard HL. Antidepressants I: Selective serotonin reuptake inhibitors. In: Martin A, Scahill L, Charney DS, et al. editors. Pediatric psychopharmacology. Oxford: Oxford Univ Press, 2003:274-83.
- Jackson J. Antianxiety drugs. In: Klykylo WM, Bowers R, Weston C, et al. editors. Green’s child and adolescent clinical psychopharmacology. Philadelphia: Lippincott Williams and Wilkins, 2014:302-17.
- Baldwin DS, Anderson IM, Nutt DJ, et al. British Association for Psychopharmacology: Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2005;19:567-96. [Crossref] [PubMed]
- Treatment for Adolescents with Depression Study (TADS) Team. The Treatment for Adolescents with Depression Study (TADS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry 2005;44:28-40. [Crossref] [PubMed]
- Emslie G, Kratochvil C, Vitiello B, et al. TADS Team. Treatment for Adolescents with Depression Study (TADS): safety results. J Am Acad Child Adolesc Psychiatry 2006;45:1440-55. [Crossref] [PubMed]
- Curry J, Rohde P, Simons A, et al. TADS Team. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 2006;45:1427-39. [Crossref] [PubMed]
- March JS, Silva S, Petrycki S, et al. The TADS Team. The Treatment for Adolescents with Depression Study (TADS): long-term effectiveness and safety outcomes. Arch Gen Psychiatry 2007;64:1132-43. [Crossref] [PubMed]
- March JS, Vitiello B. Clinical messages from the Treatment for Adolescents with Depression Study (TADS). Am J Psychiatry 2009;166:1118-23. [Crossref] [PubMed]
- Vitiello B, Silva SG, Rohde P, et al. Suicidal events in the Treatment for Adolescents with Depression Study (TADS). J Clin Psychiatry 2009;70:741-7. [Crossref] [PubMed]
- Kennard BD, Silva SG, Tonev S, et al. Remission and recovery in the Treatment for Adolescents with Depression Study (TADS): acute and long-term outcomes. J Am Acad Child Adolesc Psychiatry 2009;48:186-95. [Crossref] [PubMed]
- March J, Silva S, Curry J, et al. Treatment for Adolescents with Depression Study (TADS) Team. The Treatment for Adolescents with Depression Study (TADS): outcomes over 1 year of naturalistic follow-up. Am J Psychiatry 2009;166:1141-9. [Crossref] [PubMed]
- Compton SN, Walkup JT, Albano AM, et al. Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health 2010;4:1. [Crossref] [PubMed]
- Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008;359:2753-66. [Crossref] [PubMed]
- The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 2001;344:1279-85. [Crossref] [PubMed]
- Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA 2004;292:1969-76. [Crossref] [PubMed]
- Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA 2008;299:901-13. [Crossref] [PubMed]
- Emslie GJ, Mayes T, Porta G, et al. Treatment of Resistant Depression in Adolescents (TORDIA): week 24 outcomes. Am J Psychiatry 2010;167:782-91. [Crossref] [PubMed]
- Vitiello B, Emslie G, Clarke G, et al. Long-term outcome of adolescent depression initially resistant to selective serotonin reuptake inhibitor treatment: a follow-up study of the TORDIA sample. J Clin Psychiatry 2011;72:388-96. [Crossref] [PubMed]
- Lexi-Drugs®. Lexi-Comp, Inc. Accessed December 15, 2016.
- Buspar (package insert). Princeton, New Jersey: Bristol-Myers Squibb Company, 2010. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/018731s051lbl.pdf
- Salazar DE, Frackiewicz E, Dockens R, et al. Pharmacokinetics and tolerability of buspirone during oral administration to children and adolescents with anxiety disorder and normal healthy adults. J Clin Pharmacol 2001;41:1351-8. [Crossref] [PubMed]
- Dowben JS, Grant JS, Froelich KD, et al. Biological perspectives: hydroxzyine for anxiety: another look at an old drug. Perspect Psychiatr Care 2013;49:75-7. [Crossref] [PubMed]
- Buitelaar JK. Miscellaneous compounds: beta-blockers and opiate antagonists. In: Martin A, Scahill L, Charney DS, et al. editors. Pediatric psychoharmacology: principles and practice. Oxford, England: Oxford University Press, 2003:353-62.
- Rynn MA, Riddle MA, Yeung PP, et al. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo-controlled trials. Am J Psychiatry 2007;164:290-300. [Crossref] [PubMed]
- Mancini C, Van Ameringen M, Oakman JM, et al. Serotonergic agents in the treatment of social phobia in children and adolescents: a case series. Depress Anxiety 1999;10:33-9. [Crossref] [PubMed]
- Mrakotsky C, Masek B, Biederman J, et al. Prospective open-label pilot trial of mirtazapine in children and adolescents with social phobia. J Anxiety Disord 2008;22:88-97. [Crossref] [PubMed]
- Hammerness PG, Vivas FM, Geller DA. Selective serotonin reuptake inhibitors in pediatric psychopharmacology: a review of the evidence. J Pediatr 2006;148:158-65. [Crossref] [PubMed]
- Hoffman EJ, Mathew SJ. Anxiety disorders: a comprehensive review of pharmacotherapies. Mt Sinai J Med 2008;75:248-62. [Crossref] [PubMed]
- Ipser JC, Stein DJ, Hawkridge S, et al. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev 2009;3:CD005170. [PubMed]
- Kapczinski F, Lima MS, Souza JS, et al. Antidepressants for generalized anxiety disorder. Cochrane Database Syst Rev 2003.CD003592. [PubMed]
- Murphy TK, Segarra A, Storch EA, et al. SSRI adverse events: how to monitor and manage. Int Rev Psychiatry 2008;20:203-8. [Crossref] [PubMed]
- Pine DS. Treating children and adolescents with selective serotonin reuptake inhibitors: how long is appropriate? J Child Adolesc Psychopharmacol 2002;12:189-203. [Crossref] [PubMed]
- Walkup J, Labellarte M. Complications of SSRI treatment. J Child Adolesc Psychopharmacol 2001;11:1-4. [Crossref] [PubMed]
- Waslick B. Psychopharmacology interventions for pediatric anxiety disorders: a research update. Child Adolesc Psychiatr Clin N Am 2006;15:51-71. [Crossref] [PubMed]
- Seidel L, Walkup JT. Selective serotonin reuptake inhibitor use in the treatment of the pediatric non-obsessive-compulsive disorder anxiety disorders. J Child Adolesc Psychopharmacol 2006;16:171-9. [Crossref] [PubMed]
- Reinblatt SP, Walkup JT. Psychopharmacologic treatment of pediatric anxiety disorders. Child Adolesc Psychiatr Clin N Am 2005;14:877-908. [Crossref] [PubMed]
- Lexicomp. Buspirone. Available online: http://online.lexi.com.ezproxy.med.wmich.edu/lco/action/doc/retrieve/docid/patch_f/6486
- Lexicomp. Propranolol. Available online: http://online.lexi.com.ezproxy.med.wmich.edu/lco/action/doc/retrieve/docid/patch_f/7576
- Buitelaar JK. Miscellaneous compounds: beta-blockers and opiate antagonists. In: Martin A, Scahill L, Charney DS, et al. editors. Pediatric psychopharmacology: principles and practice. Oxford, England: Oxford University Press, 2003:353-62.
- Witek MW, Rojas V, Alonso C, et al. Review of benzodiazepine use in children and adolescents. Psychiatr Q 2005;76:283-96. [Crossref] [PubMed]
- Barnett S, Riddle MA. Anxiolytics: benzodiazepines, bupspirone and others. In: Martin A, Scahill L, Charney DS, et al. editors. Pediatric psychopharmacology: Principles and practice. Oxford: Oxford Univ Press, 2003:341-52.


