Recovery of steroid induced adrenal insufficiency
Introduction
Secondary adrenal insufficiency can result from inadequate stimulation of the adrenal glands due to either insufficiency or inadequate secretion of adrenocorticotropic hormone (ACTH). This may occur due to a variety of reasons including hypothalamic defects, hypopituitarism, defects in synthesis and processing of ACTH and chronic glucocorticoid use. The mortality and morbidity associated with secondary adrenal insufficiency depends on the underlying etiology. However, missing the diagnosis could lead to detrimental consequences.
In addition to being used as a replacement therapy for adrenal insufficiency, glucocorticoids have been widely used for their anti-inflammatory and pharmacological effects in a variety of medical conditions. The hypothalamic-pituitary-adrenal (HPA) axis can be suppressed after a single dose of steroid, but typically recovers quickly. With chronic glucocorticoid, recovery of HPA axis might take longer. Understanding the timeline for recovery of the HPA axis and tests used to assess adrenal function and the HPA axis is crucial to both avoiding missing a diagnosis of adrenal insufficiency and the use of unnecessary steroids for replacement therapy.
Underlying mechanism of steroid induced adrenal insufficiency
Steroidogenesis is controlled by multiple factors including the HPA axis. ACTH, secreted by the anterior pituitary gland, stimulates synthesis of cortisol and androgens in the adrenal cortex. ACTH is also thought to have an effect on stimulating growth and maturation of the adrenal gland. In the absence of ACTH, the adrenal glands undergo atrophy (1).
The HPA axis, like many endocrine systems, is a classic example of the feedback system (Figure 1). Glucocorticoids, whether endogenous or exogenous, exert negative feedback inhibition at the pituitary and hypothalamus levels. The glucocorticoid effects may be divided into acute and delayed phases. The acute phase usually occurs within minutes after administration. This is related to the rate of rise in cortisol level, and inhibits the release of ACTH and CRF from secretory granules. The delayed phase, on the other hand, occurs after 2–20 h and extends up to days. This phase is mainly related to the inhibition of gene transcription factors of the pro-opiomelanocortin (POMC), leading to decreased synthesis of ACTH. The delayed phase is dependent on the dose and duration of glucocorticoid use but typically occurs with chronic glucocorticoid use. This explains the fact that although only one or two doses of glucocorticoid are sufficient to suppress the HPA axis, the recovery is usually rapid. On the other hand, with prolonged use of glucocorticoids, the recovery of the HPA axis is much delayed (1,2).
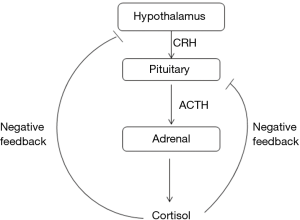
Timing of recovery of HPA function
Glucocorticoids have been used for their anti-inflammatory and pharmacological effects in multiple different disease entities including rheumatologic disorders, asthma, oncological disorders and many others. There are different studies that looked into effect of exogenous chronic glucocorticoid use on the HPA axis, and timing of recovery. However, depending on the glucocorticoids formulation used, the duration of therapy, the use of a variety of weaning protocols and the different diagnostic tests used to assess adrenal function, it is rather difficult to draw general conclusions. In this review, we will focus on a few most common medical conditions for which glucocorticoids are encountered in children.
Glucocorticoid use in childhood leukemia
Glucocorticoids have been widely used in the treatment of childhood leukemia. These patients are at a high risk for infectious complications, which makes identification of secondary adrenal insufficiency in these children critical. There are multiple studies that looked into the effect of chronic glucocorticoid use on the HPA axis and timing of recovery in this population of patients. Felner et al. (3), evaluated 10 children with B cell acute lymphoblastic leukemia (ALL) who received 28 day-course of high dose dexamethasone. The recovery of adrenal function was assessed by performing a high dose (250 µg) ACTH stimulation test. All patients were noted to have testing consistent with adrenal insufficiency on day 1 after cessation of glucocorticoid therapy. Time for recovery of the HPA axis ranged from 4–8 weeks. Mahachoklertwattana et al. (4), evaluated children with ALL who received induction therapy with prednisone for 28 days followed by 7 days of dexamethasone for 4 weeks. Low dose ACTH stimulation test was used to assess recovery of HPA axis. Most patients showed recovery of the HPA axis by 4–12 weeks. However, 13% of patients had persistent adrenal insufficiency at 20 weeks after receiving glucocorticoid therapy. Einaudi et al. (5), evaluated patients in two arms of glucocorticoid therapy. One arm received 22 days of prednisone tapering over 9 days and the second group received 22 days of dexamethasone which was weaned over 9 days. All patients demonstrated recovery of HPA axis by 10 weeks. Based on the above studies, it appears that most patients demonstrated recovery of HPA axis between 4–10 weeks after cessation of therapy.
Glucocorticoid use in hemangioma
Glucocorticoids are widely used for treatment of hemangiomas in infants. Mendoza-Cruz et al. (6) looked into duration of HPA axis suppression in infants treated with prednisolone. The infants received high dose of prednisolone for an average of 3–6 months, weaned over 4–6 weeks. Salivary cortisol was used to assess recovery of circadian rhythm, as this would be the first sign of recovery of HPA axis. Circadian rhythm was noted to be established by 6 weeks after cessation of oral steroids. That was confirmed by low dose (1 µg) ACTH stimulation test. All infants recovered and none had persistent adrenal insufficiency by 10–12 weeks after cessation of glucocorticoid therapy.
Glucocorticoid and childhood asthma
Inhaled corticosteroids (ICS) have been widely used in the treatment of persistent asthma for a long time. It was believed that when used within recommended doses, the risk for clinically relevant adrenal suppression is minimal (7). However, with increasing case reports of adrenal insufficiency with the use of ICS, the importance of understanding the risk factors associated with HPA suppression have become more obvious. In fact, some studies have shown that as many as 20–50% of children treated with ICS had biomedical abnormalities of the HPA axis (8). Fluticasone propionate is much more likely to cause clinically symptomatic adrenal suppression compared to other ICS like beclomethasone, triamcinolone and budesonide. Dose of fluticasone of 352 µg per day were noted to result in adrenal suppression in about 50% of patients (9). Eid et al. evaluated children with asthma who were treated with inhaled fluticasone propionate (10). These patients had early morning cortisol levels assessed after using the medication for 3–13 months. Seventeen percent of patients on low dose ICS had morning cortisol levels of less than 5.5 µg/dL compared to 45% of children on the high dose. Those children who were switched to different formulations or lower doses were noted to demonstrate improvement in cortisol levels after changing the medication.
Breborowicz et al. evaluated patients with severe asthma receiving recommended doses of ICS of 500–1,000 µg/day of fluticasone propionate or the equivalent of budesonide (1,000–2,000 µg/day) for a period of at least 12 months. These children had their adrenal function evaluated by early morning cortisol and low dose ACTH stimulation test. None of the patients demonstrated any evidence of adrenal suppression (11).
The current guidelines for screening for adrenal insufficiency in the setting of ICS use recommend screening high risk patients including: chronic use of moderate to high dose of high potency ICS for longer than 6 months; concurrent use of inhaled or topical corticosteroids; frequent medication with oral glucocorticoids, and low body mass index (BMI) (12). Screening may be done by early morning cortisol level and confirmation with low dose ACTH stimulation test if cortisol level is less than10 µg/dL (13,14).
Dynamic testing of HPA axis
There are multiple provocative tests which may be used for evaluation of the HPA axis. These provocative agents test different levels of the HPA axis. Insulin tolerance test (ITT) has long been considered the gold standard as it assesses the integrity of the full HPA axis. However, ITT has not been widely used in many institutions given the risk of hypoglycemia and the usual presence of multiple contraindications. In addition, it can be resource intensive and expensive (15). Metyrapone stimulation test is considered to be a sensitive test and comparable to ITT since it evaluates integrity of the HPA axis as a whole. It is based on the fact that metyrapone inhibits 11 hydroxylases, leading to decreased cortisol level, and thus stimulating the pituitary secretion of ACTH. The use of this test has been challenging due to unavailability of metyrapone and risk of inducing an adrenal crisis. The variation in ACTH assays and availability of 11 deoxycortisol assays make this test a suboptimal modality for testing in these children (16,17).
ACTH stimulation test provides an indirect assessment of adrenal cortical function in the state of chronic ACTH deficiency. The high dose ACTH stimulation test (HDST) (250 µg) utilizes supra-physiologic doses of ACTH sufficient to stimulate atrophied adrenal glands, thus leading to false negative results. The low dose ACTH stimulation test (LDST) (1 µg) was believed to be an appropriate solution this concern. However, there are some challenges in regards to techniques for dilution of the ACTH preparation due to lack of standardized protocols and cut off thresholds. This has led to controversies about the accuracy value of the test. Multiple studies comparing low dose with high dose ACTH stimulation tests found that LDST has higher sensitivity but lower specificity compared to the HDST (18). Suggestions have been made about increasing the cut off range for HDST to improve sensitivity of the test. Giordano et al. studied the sensitivity and specificity of different provocative tests in comparison to ITT in adults. They found that HDST cortisol cutoff of 37 µg/dL would make the test 95% sensitive. For best pairs of values for highest sensitivity and specificity, they suggested a cortisol cutoff of 21 µg/dL for HDST compared to 17 µg/dL for the LDST (19).
The use of glucagon as a provocative agent for assessment of HPA axis has also been studied. Its use in conjunction with growth hormone (GH) assessment in GH provocative testing protocols makes it particularly useful. Bottner et al. reported that glucagon stimulation test was comparable to testing with CRH and ITT in children. They suggested cortisol cutoff of 16 µg/dL for best pair of sensitivity and specificity (20). Another cortisol cutoff was suggested at 14.6 compared to 20 µg/dL for ITT (21).
Conclusions
The widespread use of glucocorticoids for their potent anti-inflammatory and pharmacological effects comes at the risk of side effects such as secondary adrenal insufficiency. Identifying patients at high risk for developing adrenal insufficiency and the appropriate testing protocols is crucial to avoid unnecessary glucocorticoid replacement on one hand, and missing a diagnosis of adrenal insufficiency with detrimental consequences on the other.
Use of exogenous glucocorticoids is known to cause suppression of the HPA axis. Secondary adrenal insufficiency may be noted with oral and inhaled glucocorticoid administration. Typically, the HPA axis recovers fairly quickly if glucocorticoids have been used for less than 10–14 days. If glucocorticoids have been used for 2 weeks or longer then weaning of steroids and assessment of the integrity of the HPA axis are recommended. In the meantime, patients should be educated about the use of steroid coverage for stress until recovery of HPA axis is documented.
The optimal time to test for HPA axis recovery following prolonged glucocorticoid use remains controversial due to variability of data for timelines of when that occurs. In general, the earliest that HPA axis recovery may be seen is about 4 weeks post-cessation of prolonged glucocorticoid use. It would be therefore reasonable to plan assessment of HPA axis around that time and then every 1–2 months until recovery is documented. Early morning 8 AM cortisol level is useful screening test especially with ICS. If early morning cortisol level is <10 µg/dL then further dynamic testing of the HPA axis needs to be considered. ITT is considered to be the gold standard but is not widely used due to risk of side effects. Metyrapone stimulation test is believed to be comparable to ITT and is a good alternative test. However, the risk of adrenal crisis and limited availability of metyrapone itself make this test less appealing. Low dose ACTH stimulation test is a highly sensitive test for secondary adrenal insufficiency. It does not evaluate recovery of the axis at the hypothalamus and pituitary level. Moreover, there are some technical concerns about the dilution techniques and lack of standardized protocols for this testing which further complicate the issue. Glucagon stimulation test is an alternative as well, but again there are different cortisol cutoff thresholds published which makes standardization difficult. Therefore, low dose ACTH stimulation test is a good test with high sensitivity to assess the HPA axis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kannan CR. The Adrenal Gland. Springer, 2011.
- Sperling MA. Pediatric Endocrinology. Fourth ed. Elsevier, 2014.
- Felner EI, Thompson MT, Ratliff AF, et al. Time course of recovery of adrenal function in children treated for leukemia. J Pediatr 2000;137:21-4. [Crossref] [PubMed]
- Mahachoklertwattana P, Vilaiyuk S, Hongeng S, et al. Suppression of adrenal function in children with acute lymphoblastic leukemia following induction therapy with corticosteroid and other cytotoxic agents. J Pediatr 2004;144:736-40. [Crossref] [PubMed]
- Einaudi S, Bertorello N, Masera N, et al. Adrenal axis function after high-dose steroid therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2008;50:537-41. [Crossref] [PubMed]
- Mendoza-Cruz AC, Wargon O, Adams S, et al. Hypothalamic-pituitary-adrenal axis recovery following prolonged prednisolone therapy in infants. J Clin Endocrinol Metab 2013;98:E1936-40. [Crossref] [PubMed]
- Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol 2011;204:479-87. [Crossref] [PubMed]
- Blair J, Lancaster G, Titman A, et al. Early morning salivary cortisol and cortisone, and adrenal responses to a simplified low-dose short Synacthen test in children with asthma. Clin Endocrinol (Oxf) 2014;80:376-83. [Crossref] [PubMed]
- Schwartz RH, Neacsu O, Ascher DP, et al. Moderate dose inhaled corticosteroid-induced symptomatic adrenal suppression: case report and review of the literature. Clin Pediatr (Phila) 2012;51:1184-90. [Crossref] [PubMed]
- Eid N, Morton R, Olds B, et al. Decreased morning serum cortisol levels in children with asthma treated with inhaled fluticasone propionate. Pediatrics 2002;109:217-21. [Crossref] [PubMed]
- Breborowicz A, Niedziela M. Adrenal function in children with severe asthma treated with high-dose inhaled glucocorticoids: recommended screening tests in outpatient conditions. J Pediatr Endocrinol Metab 2007;20:781-9. [Crossref] [PubMed]
- Zollner EW, Lombard CJ, Galal U, et al. Hypothalamic-pituitary-adrenal axis suppression in asthmatic school children. Pediatrics 2012;130:e1512-9. [Crossref] [PubMed]
- Issa-El-Khoury K, Kim H, Chan ES, et al. CSACI position statement: systemic effect of inhaled corticosteroids on adrenal suppression in the management of pediatric asthma. Allergy Asthma Clin Immunol 2015;11:9. [Crossref] [PubMed]
- Lougheed MD, Lemiere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J 2012;19:127-64. [Crossref] [PubMed]
- Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab 1994;79:923-31. [PubMed]
- Steiner H, Bahr V, Exner P, et al. Pituitary function tests: comparison of ACTH and 11-deoxy-cortisol responses in the metyrapone test and with the insulin hypoglycemia test. Exp Clin Endocrinol 1994;102:33-8. [Crossref] [PubMed]
- Spiger M, Jubiz W, Meikle AW, et al. Single-dose metyrapone test: review of a four-year experience. Arch Intern Med 1975;135:698-700. [Crossref] [PubMed]
- Ng SM, Agwu JC, Dwan K. A systematic review and meta-analysis of Synacthen tests for assessing hypothalamic-pituitary-adrenal insufficiency in children. Arch Dis Child 2016;101:847-53. [Crossref] [PubMed]
- Giordano R, Picu A, Bonelli L, et al. Hypothalamus-pituitary-adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparison of different provocative tests. Clin Endocrinol (Oxf) 2008;68:935-41. [Crossref] [PubMed]
- Bottner A, Kratzsch J, Liebermann S, et al. Comparison of adrenal function tests in children--the glucagon stimulation test allows the simultaneous assessment of adrenal function and growth hormone response in children. J Pediatr Endocrinol Metab 2005;18:433-42. [Crossref] [PubMed]
- di Iorgi N, Napoli F, Allegri A, et al. The accuracy of the glucagon test compared to the insulin tolerance test in the diagnosis of adrenal insufficiency in young children with growth hormone deficiency. J Clin Endocrinol Metab 2010;95:2132-9. [Crossref] [PubMed]


