Current surgical strategies and techniques of aortic valve diseases in children
Introduction
The surgical techniques and innovations that can be applied to the treatment of aortic valve disease have recently increased, and the choice of different therapeutic methods is influenced by many factors, such as economic considerations, proficiency in a particular technique and the choice of the patient’s family (1). The ultimate goal of these aortic repair or reconstruction techniques is to provide the best survival, minimal reintervention risk, and high quality of life. From this point of view, the purpose of this article is to analyze the current surgical strategies and techniques of treating aortic valve disease in children.
Classification of aortic valve disease in children
Aortic valve pathology in children mainly includes congenital aortic valve anomalies and acquired aortic valve diseases. Congenital aortic valve anomalies are caused by abnormal leaflet and/or annular morphology which result in stenosis, insufficiency or both. Diseased aortic leaflets can be bicuspid (the most common), tricuspid (trileaflet), unicuspid, or quadricuspid. The bicuspid aortic valve can be considered as benign for most patients as they do not result in significant functional problems. However, when bicuspid aortic valve results in critical aortic stenosis, multiple interventions are required (2-4). Aortic valve disease can also be secondary to other forms of congenital heart disease, such as leaflet prolapse in the setting of a ventricle septal defect (5).
Acquired aortic valve disease include rheumatic heart disease (RHD) and subacute bacteria endocarditis (SBE) which usually cause leaflet thickening, commissural fusion, vegetation and leaflet destruction. In the current era, aortic valve disease secondary to RHD and SBE are infrequent. RHD can affect one or more heart valves after an episode of acute rheumatic fever (ARF), and leaflets become stretched or scarred resulting in obstruction of blood flow. Malformed stenotic or regurgitant valves may be subacutely infected during episodes of bacteremia, likely via initial adhesion and subsequent colonization of the surface area. Less commonly, subacute bacterial endocarditis can affect the healthy heart valve (6-8).
What is the primary procedure of choice in children with aortic valve stenosis?
Treatment of congenital aortic valve stenosis usually requires multiple interventions. The ultimate goal of treatment should result in the adequate relief of obstruction while minimizing significant regurgitation. Both balloon aortic valvuloplasty (BAV) and surgical aortic valvotomy (SAV) have been widely applied, but the primary intervention for congenital aortic valve stenosis in children still remains controversial (9).
In 2015, Prijic and colleagues (10) reported a study that evaluated the long-term results of BAV (n=39, 1.3 months to 17 years old) and SAV (n=23, 1.2 months to 15 years old) from 1987 to 2013. The freedom from reintervention rates were 71% for SAVs and 61% for BAV in 10 years, but 42% for SAV and 23% for BAV in 20 years. These results reveal that the long-term outcome of SAV is better that that of BAV, though the early results might look like less different. Not surprisingly, freedom from aortic valve replacement (AVR) for BAV (32%) is lower than that for SAV (53%). But no matter what type of surgery we choose, congenital aortic stenosis carries the lifelong risk of reintervention.
In 2015, Soulatges and colleagues (11) analyzed 93 patients (1 day to 18 years, 2.4 years on average) who underwent BAV as the primary treatment from 1992 to 2012. The long-term survival rate was 88.2% in whole cohort. The freedom from reintervention rate was 58% (54% in neonates and 65.6% in infants) with the mean follow-up duration of 11.4 years and the freedom from surgery was 66% (58.5 in neonates and 75.8 in infants). The authors concluded that BAV was efficient and low-risk when applied as the primary treatment in infants and children. While in 2006, Miyamoto and colleagues (12) reported a freedom from reintervention of 85.1%, 78.0%, and 53.5% at 5, 10, and 15 years respectively in patients who underwent primary SAV due to the critical aortic stenosis aged less than 3 months between 1983 and 2003 and they concluded that SAV was the most appropriate strategy to treat neonates and young infants with critical aortic stenosis. Collectively, these data suggest that the outcomes of BAV in younger patients were no better, if not worse, than those of SAV. In reference to a recent meta-analysis (13), we can intuitively see that SAV and BAV had similar early mortality, but the long-term outcomes (the freedom from reintervention) of SAV were superior overall and in infant alone (P<0.001).
Aortic valve repair techniques
With the development of valve repair techniques, aortic valve repair shows several advantages over replacement. On the one hand, repair can be performed in almost all ages and utilizes native tissue, do not require anticoagulation, and has a low risk of endocarditis, calcification or thromboembolism. Furthermore, valve repair stabilizes left ventricular dimensions, hemodynamics and results in symptomatic improvement, and various strategies can be utilized as the patient grows. Surgical repair of aortic valve can be classified as simple or complex. Simple methods include valvulotomy, valve debridement, commissurotomy, valve suspension, and commissure suspension. Complex aortic valve repair methods include cusp extension, leaflet replacement, and other valve reconstruction. Extensive valve debridement, nodular fibrosis resection, fused commissures opening and thickened cusp areas thinning are usually involved in the treatment of stenotic or rheumatic aortic valve disease (14,15).
Aortic valve repair without using exogenous patch material
Valvulotomy requires one or more incisions at the edges of the commissure to relieve aortic valve constriction. As the first step in the treatment of aortic valve stenosis in children, valvulotomy is a palliative and therapeutic method. Vobecky and colleagues first reported their surgical experience of aortic stenosis including trans-ventricular and trans-aortic valvulotomy in 1991. The overall survival rate was 75% in 5 years and 4 patients that had undergone valvotomy via the trans-ventricular approach died. The trans-ventricular valvulotomy was listed as a risk factor for death with, in addition to preoperative hemodynamic state, surgical weight and associated anomalies of the left ventricle (16). Duro and colleagues reported their results of aortic valvotomy using cardiopulmonary bypass and moderate hypothermia in 22 patients (mean age was 7.3±3.6 years) with a follow-up of 8.6±5.4 years. They demonstrated no more than mild regurgitation, no mortality and seven reinterventions after aortic valvotomy. Further, valve replacement was avoided or at least postponed until childhood (17). But reintervention is still likely needed later in life.
Valve debridement is widely used in aortic valve repair which clears the infected or thickened portion of the aortic valve leaflet (18). Infective endocarditis not responsive to antibiotic therapy require surgical debridement where all infected leaflet, annular and myocardial tissues are removed (19,20).
Debridement commonly results in aortic valve tissue deficiency and thus requires reconstruction during the same procedure. To reconstruct the aortic valve, the placement of patch material is needed (see below), but sometimes this can be treated with primary closure of the defect followed by the suspension of the leaflet by commissural plication to permit adequate coaptation of the three cusps, and distribute leaflet stress.
In some cases, stenosis requires the opening and suspension of a commissure. In patients with redundant tissue causing prolapse, leaflet plication and commissuroplasty can be applied to shorten leaflet length and reduce prolapse (14).
Aortic valve repair by using patch material
Leaflet extension extends the aortic valve leaflets to the level of the sinotubular junction to establish or enlarge the surface of coaptation. Extension can be accomplished by using different patch materials. Autologous pericardium treated with glutaraldehyde is thought to be a suitable material but with the risk of pericardial inflammation, while fresh autologous pericardium gradually loses pliability due to fibrous degenerative changes (21). Bianchi (22) published his view that autologous pericardium is better than conventional bovine pericardium in congenital heart disease reconstructive surgery. Furthermore, animal experiments show that fixed autologous pericardium is superior to foreign pericardium in resisting calcification which goes against the long-term durability (23). The search for the ideal patch material for valve repair and reconstruction is still ongoing.
During leaflet extension, three stay sutures should be placed at the lower and upper flaps of the aortotomy in order to expose the aortic valve so that annulus diameter and leaflet free edge length can be measured accurately. After inspection of all aortic cusps, patches are cut according to the characteristics of aortic, such as valve morphology, commissural fusion or cusp prolapse. The fixed patches are finally sutured on the aortic wall and free edges of native valves. Care should be taken to avoid coronary artery obstruction.
The leaflet extension technique has become one of the most frequently used aortic valve repair procedures in the pediatric population because of its ease and reliability. Kalangos and colleagues (24) used leaflet extension to repair the aortic valve in children with rheumatic aortic disease. They reported only one early death and one late death occurred during the follow-up of 77 children who underwent cusp extension and a freedom from aortic valve reoperation was 88.5%, 81.7% and 79.7% at 5, 10 and 15 years, respectively. They concluded that cusp extension remains a suitable transitional approach before later AVR (19,25,26).
Aortic valve reconstruction was first reported by Dr. Duran (27). His procedure attempted to reproduce tailored valves as much as possible. They used three consecutive bulges of different sizes as templates to guide the shaping of the pericardium, which was made according to the dimensions of the aortic annulus. The pericardial leaflets were sutured to the aortic valve remnant or to the annulus (28). Duran shared the experience of 51 patients who underwent Duran procedure with the survival rate of 84.53% at the follow-up of 60 months, and 72.59% of the patients was free from any event after the operation. He concluded that early outcomes encouraged us to reconstruct the aortic valve by using autologous pericardium.
Aortic leaflet replacement was developed by Professor Shigeyuki Ozaki nearly a decade ago. The Ozaki procedure consists of using glutaraldehyde-treated autologous pericardium to replace aortic valve leaflets but improves significantly in measuring the diseased valve and using templates to cut fixed pericardium compared with the duran procedure. As no foreign material is required, postoperative anticoagulation is unnecessary, and the Ozaki procedure was thus considered as valve repair rather than replacement (29). First step of the procedure is to prepare the pericardium, including cleaning fat and other redundant tissue, treated it with 0.6% glutaraldehyde solution for 10 minutes and then rins 3 times using physiologic saline solution. The diseased cusps are excised meticulously. Next is to measure the distance between each commissure and trim the pericardium. Special instruments were developed by Dr. Ozaki including a sizer head and a template. The sizer is used to measure the diseased part of the valve. The template is used to cut replacement tissue to the exact size and shape and place dot as marking to ensure the pericardial leaflets are sewn precisely into place which allows the valve to function in its normal way. Last, the clipped pericardial cusp is sutured to each annulus with smooth (inner) surface on the proximal side. Shigeyuki Ozaki himself reported a study of 404 case who underwent Ozaki procedure (289 of stenosis and 115 of regurgitation) with freedom from reoperation of 96.2% and he considered it was feasible for appropriate patients (30). He published the early follow-up of the patient who underwent Ozaki procedure with the unicuspid, bicuspid, or quadricuspid aortic valve respectively and glutaraldehyde-treated autologous pericardium performed well with good hemodynamics and without anticoagulation. But lack of a control group and short follow-up need were shortcomings of this study (31-33).
The majority of cases reported by Dr. Ozaki were adult, now many pediatric cardiac surgeons applied this technique in pediatric cases, and they also achieved satisfactory results (34,35).
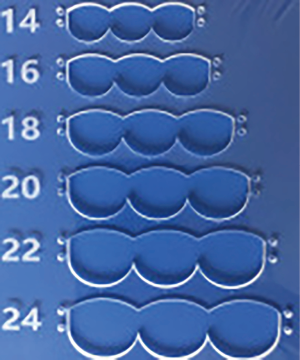
We have developed a novel technique of aortic valve reconstruction by using glutaraldehyde-treated autologous pericardium. This technique differs from the Ozaki procedure in that we use a single piece of fixed pericardium as opposed to three separate leaflets (Figure 1). We have applied this novel technique in five children. Three had aortic stenosis, three had aortic insufficiency, and one patient had both stenosis and insufficiency. A custom-made template that was initially designed for hand making ePTFE valved conduit (34) was used to trace the shape of aortic valve leaflets (in continuity) on autologous pericardium (Figure 1). The aortic annulus was then divided in three equal parts to match the prefabricated leaflets. A suture line with 5-0 or 6-0 polypropylene was started from the midpoint of each cusp ensuring the left and right coronary ostia were positioned in the center of the cusp. At the three leaflet junctions, pledgeted stiches were placed from inside to outside of the aortic wall to create three neocommissures (Figure 2). Postoperative transesophageal echocardiogram usually demonstrated mobile leaflets and ample length of coaptation zone with no obstruction or regurgitation. Early follow-up has revealed a persistent excellent result (Figure 3). A 4-month-old boy with severe aortic insufficiency due to infectious endocarditis underwent aortic valve reconstruction by using autologous pericardial patch. Aortic competence was achieved with a 25-mmHg pressure gradient. Compared to the Ozaki procedure, we believe this procedure is easy to manipulate especially during making commissures and it may help to standardize the surgical procedure, but further follow-up and increased experience with this technique are needed.
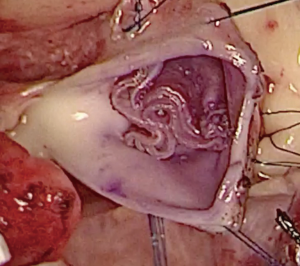
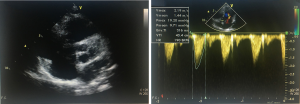
Technique of AVR
Although aortic valve repair in children has developed rapidly, AVR may still be unavoidable in some cases (36). Ideally, desired substitutes of the aortic valve need to have the following characteristics: long-term durability, lack immunogenecity, require no anticoagulation, and have the ability to grow. In other words, they need to be comparable with normal native valves (15). There are four replacement options based on their material composition: pulmonary autograft, mechanical prosthesis, aortic allograft (homograft), and bioprosthetic valve (37). Each has its advantages and limitations and there is no ideal substitute in children because none are identical to a native valve when considering the long-term results of AVR (38).
The Ross procedure, first reported by Ross in 1967, utilizes the patient’s own pulmonary valve and root to replace a diseased aortic valve (39). A right ventricle to pulmonary artery conduit is then used to reconstruct the right ventricle outflow tract. It is the only procedure that provides a living valve substitute and there are obvious advantages in terms of avoiding long-term anticoagulation, growth potential, and low risk of endocarditis (40). But reintervention for the pulmonary autograft and right ventricle to pulmonary artery conduit may be required after such a complex surgical procedure. Brancaccio and colleagues (41) reported their experience of 55 children who underwent a Ross procedure from 1993 to 2012. The overall survival was 84.9% during the 10-year follow-up and freedom from any reoperation was 48.1%. Five patients with severe aortic insufficiency and one with aortic root dilatation required reoperation on the autograft. They concluded that the Ross procedure is an attractive option in children for the complex left ventricular outflow tract obstruction (LVOTO) and aortic valve disease due to its low rate of autograft failure but may be not suitable in older patients. In 1975, Konno and colleagues (42) described a method to enlarge the aortic root and it is the preferred treatment for multilevel LVOTO in children. Ruzmetov and colleagues (43) concluded from 78 children underwent the Ross procedure (including 18 with Ross-Konno procedure) between 1993 and 2011 that the risk of common postoperative complications (autograft insufficiency and right ventricular outflow tract obstruction, which may require reoperation) were not significantly different, though the mortality of Ross-Konno procedure was much higher than that of the Ross procedure due to the preoperative complexity.
Mechanical valves perform well in the long-term follow-up and are easier to implant. But in children the bleeding complications caused by lifelong anticoagulation and the size mismatch between prostheses and growing patients are still unsolved problems (44). Kato and colleagues (45) reported a study on AVR with small mechanical valves (<19 mm). Seventy-eight patients (86% aged ≥65 years) who underwent AVR with 16 mm (n=21), 17 mm (n=25), or 18 mm (n=32) valves achieved satisfactory early and mid-term results. One hospital and four cardiac-related deaths occurred and the left ventricular mass was decreased significantly. But smaller valves seem to be associated with higher postoperative pressure gradient. The authors concluded that small mechanical prostheses may be an acceptable method for elderly patients with a small aortic root. However the translatability of these results to children remains in question.
The aortic allograft has been considered an option for patients who need complex reconstruction of the aortic root or those who are too small for mechanical or bioprosthetic valves. The obvious advantages of the aortic allograft are good hemodynamics, low thrombogenicity, and no anticoagulation. However early calcification and lack of availability limit their widespread adoption (46).
Bioprosthesis valves perform similar to aortic allografts, and it is more readily available. But there have been some concerning reports in recent studies of early calcification, early degeneration and structural failure, especially in young patients. Thus, the improvement of materials technology and adequate long-term follow-up of cases are needed in aortic bioprosthesis for children (47).
The advantages and disadvantages of the different options of AVR are still under debate. Etnel and colleagues reported a meta-analysis of the Ross procedure (n=2,409, 9.4 years old on average), mechanical valve replacement (n=696, 12.8 years old on average) and homograft valve replacement (n=224, 8.9 years old on average) with mean follow-up of 6.6 years. The mortality was 4.20%, 7.34% and 12.82% for the Ross procedure, mechanical valve and homograft valve in early follow-up, respectively. The late annual mortality of the Ross procedure (0.46%) was still lower than that of mechanical (1.23%) and homograft (1.59%) valve. The reoperation rates of homograft valve replacement (5.44%/y) were significantly higher compared with that of the Ross procedure (1.60%/y) and mechanical valve replacement (1.07%/y). The authors concluded that the outcomes of all currently available substitutes are suboptimal, and novel solutions with durable repair techniques are in urgent need (48).
The diseased aortic valve may not be repaired or replaced with the techniques mentioned above because the annulus may be too small which prevent the use of prosthesis and pulmonary valvar or coronary malformations may be the contraindication of the Ross procedure. Thus, aortic leaflet replacement with fixed autologous pericardium may be the most appropriate for these challenging cases.
Conclusions
There is no doubt that valve repair should be first considered when treating aortic valve disease in children, but replacement may be unavoidable in some cases. Aortic valve repair can be performed at almost all ages. It is associated with low operative mortality and low risk of thromboembolism. It offers good relief of symptoms and hemodynamic improvement allowing the child to grow. Some authors believe that durability of repair is higher in patients who do not require cusp extension or use of patch material. But recent literature demonstrated that complex and simple repairs have similarly good long-term outcomes although the using of patch material during repair used to be thought with lower freedom from reintervention. Aortic valve repair does not preclude future repair or replacement procedures.
The Ozaki procedure has become more widely adopted with the advent of leaflet templates, thereby making the technique more standardized and reproducible. The Ozaki procedure achieved excellent result in large series of patients, but pediatric cases accounted a small proportion and further follow-up is needed particularly in the pediatric population.
In neonates and small infants with severe aortic stenosis, BAV and SAV provide comparable survival and freedom from reintervention, but the need for reintervention is high with both modalities. In the overall pediatric population, the outcomes are difficult to interpret due to great variations in ages in the current studies. So the primary procedure either BAV or SAV depends on institutional experiences and proficiency.
Ross or Ross-Konno should be applied in neonates, infants and small children with valvar or subvalvar AS requiring AVR. Lower mortality, valve-related complications, and better hemodynamics were seen after the Ross procedure than the other types of AVR prostheses. The Ross procedure remains the treatment of choice for children who need an AVR and have an adequate pulmonary valve. Mechanical prosthesis only can be applied in those with adequate aortic annulus or abnormal pulmonary valve and have reached their full growth potential. Bioprosthesis is rarely used due to the high incidence of calcification and degeneration.
Acknowledgements
None
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- d’Udekem Y. Aortic valve repair in children. Ann Cardiothorac Surg 2013;2:100-4. [PubMed]
- Copstead-Kirkhorn LE, Lee-Ellen C, Banasik JL, et al. Pathophysiology. Elsevier Health Sciences, 2014.
- Cripe L, Andelfinger G, Martin LJ, et al. Bicuspid aortic valve is heritable. J Am Coll Cardiol 2004;44:138-43. [Crossref] [PubMed]
- Han RK, Gurofsky RC, Lee KJ, et al. Outcome and growth potential of left heart structures after neonatal intervention for aortic valve stenosis. J Am Coll Cardiol 2007;50:2406-14. [Crossref] [PubMed]
- Sbizzera M, Pozzi M, Cosset B, et al. Long-term complications after surgical correction of Laubry-Pezzi syndrome. J Thorac Dis 2016;8:E232-4. [Crossref] [PubMed]
- Liu M, Lu L, Sun R, et al. Rheumatic Heart Disease: Causes, Symptoms, and Treatments. Cell Biochem Biophys 2015;72:861-3. [Crossref] [PubMed]
- Marijon E, Mirabel M, Celermajer DS, et al. Rheumatic heart disease. Lancet 2012;379:953-64. [Crossref] [PubMed]
- Kiyota Y, Della Corte A, Montiero Vieira V, et al. Risk and outcomes of aortic valve endocarditis among patients with bicuspid and tricuspid aortic valves. Open Heart 2017;4:e000545. [Crossref] [PubMed]
- Donald JS, Konstantinov IE. Surgical Aortic Valvuloplasty Versus Balloon Aortic Valve Dilatation in Children. World J Pediatr Congenit Heart Surg 2016;7:583-91. [Crossref] [PubMed]
- Prijic SM, Vukomanovic VA, Stajevic MS, et al. Balloon dilation and surgical valvotomy comparison in non-critical congenital aortic valve stenosis. Pediatr Cardiol 2015;36:616-24. [Crossref] [PubMed]
- Soulatges C, Momeni M, Zarrouk N, et al. Long-Term Results of Balloon Valvuloplasty as Primary Treatment for Congenital Aortic Valve Stenosis: a 20-Year Review. Pediatr Cardiol 2015;36:1145-52. [Crossref] [PubMed]
- Miyamoto T, Sinzobahamvya N, Wetter J, et al. Twenty years experience of surgical aortic valvotomy for critical aortic stenosis in early infancy. Eur J Cardiothorac Surg 2006;30:35-40. [Crossref] [PubMed]
- Hill GD, Ginde S, Rios R, et al. Surgical Valvotomy Versus Balloon Valvuloplasty for Congenital Aortic Valve Stenosis: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2016;5. [Crossref] [PubMed]
- Alsoufi B, d'Udekem Y. Aortic valve repair and replacement in children. Future Cardiol 2014;10:105-15. [Crossref] [PubMed]
- Khan MS, Samayoa AX, Chen DW, et al. Contemporary experience with surgical treatment of aortic valve disease in children. J Thorac Cardiovasc Surg 2013;146:512-20; discussion 520-1. [Crossref] [PubMed]
- Vobecky SJ, Chartrand C, Yangni-Angaté H, et al. Critical aortic stenosis in newborn infants. Twenty-five years' experience. Ann Chir 1991;45:756-9. [PubMed]
- Cabrera Duro A, López Fernández Y, Martínez Corrales P, et al. Aortic valve stenosis. Surgical treatment in children. An Esp Pediatr 1997;46:555-60. [PubMed]
- Craver JM. Aortic valve debridement by ultrasonic surgical aspirator: a word of caution. Ann Thorac Surg 1990;49:746-52; discussion 752-3. [Crossref] [PubMed]
- Hussain ST, Witten J, Shrestha NK, et al. Tricuspid valve endocarditis. Ann Cardiothorac Surg 2017;6:255-61. [Crossref] [PubMed]
- Hosseini S, Rezaei Y, Mazaheri T, et al. Tricuspid Valve Repair for Infective Endocarditis with Periannular Involvement: Complete Valve Reconstruction. J Heart Valve Dis 2016;25:730-8. [PubMed]
- Liao K, Frater RW, LaPietra A, et al. Time-dependent effect of glutaraldehyde on the tendency to calcify of both autografts and xenografts. Ann Thorac Surg 1995;60:S343-7. [Crossref] [PubMed]
- Bianchi G. eComment. Autologous pericardium is superior to conventional bovine patch in congenital heart disease reconstructive surgery: an appraisal for tissueengineered xenograft. Interact Cardiovasc Thorac Surg 2013;17:702-3. [Crossref] [PubMed]
- Jiang WJ, Cui YC, Li JH, et al. Is autologous or heterologous pericardium better for valvuloplasty? A comparative study of calcification propensity. Tex Heart Inst J 2015;42:202-8. [Crossref] [PubMed]
- Kalangos A, Myers PO. Aortic cusp extension for surgical correction of rheumatic aortic valve insufficiency in children. World J Pediatr Congenit Heart Surg 2013;4:385-91. [Crossref] [PubMed]
- Brown JW, Patel PM, Ivy Lin JH, et al. Ross Versus Non-Ross Aortic Valve Replacement in Children: A 22-Year Single Institution Comparison of Outcomes. Ann Thorac Surg 2016;101:1804-10. [Crossref] [PubMed]
- d'Udekem Y. Aortic valve surgery in children. Heart 2011;97:1182-9. [Crossref] [PubMed]
- Duran CM, Gometza B, Kumar N, et al. Aortic valve replacement with freehand autologous pericardium. J Thorac Cardiovasc Surg 1995;110:511-6. [Crossref] [PubMed]
- Duran CM, Gallo R, Kumar N. Aortic valve replacement with autologous pericardium: surgical technique. J Card Surg 1995;10:1-9. [Crossref] [PubMed]
- Ozaki S, Kawase I, Yamashita H, et al. Aortic valve reconstruction using self-developed aortic valve plasty system in aortic valve disease. Interact Cardiovasc Thorac Surg 2011;12:550-3. [Crossref] [PubMed]
- Ozaki S, Kawase I, Yamashita H, et al. A total of 404 cases of aortic valve reconstruction with glutaraldehyde-treated autologous pericardium. J Thorac Cardiovasc Surg 2014;147:301-6. [Crossref] [PubMed]
- Ozaki S, Kawase I, Yamashita H, et al. Reconstruction of bicuspid aortic valve with autologous pericardium--usefulness of tricuspidization. Circ J 2014;78:1144-51. [Crossref] [PubMed]
- Kawase I, Ozaki S, Yamashita H, et al. Aortic valve reconstruction of unicuspid aortic valve by tricuspidization using autologous pericardium. Ann Thorac Surg 2012;94:1180-4. [Crossref] [PubMed]
- Kawase I, Ozaki S, Yamashita H, et al. Original aortic valve plasty with autologous pericardium for quadricuspid valve. Ann Thorac Surg 2011;91:1598-9. [Crossref] [PubMed]
- Zhang HF, Ye M, Yan XG, et al. Application of a Simplified Hand-Sewn Trileaflet Valved Conduit in Right Ventricular Outflow Tract Reconstruction as an Alternative for Bovine Jugular Vein Graft: Single-Center Experience. Artif Organs 2018;42:41-8. [Crossref] [PubMed]
- Hosseinpour AR, González-Calle A, Adsuar-Gómez A, et al. A simple method of aortic valve reconstruction with fixed pericardium in children. Interact Cardiovasc Thorac Surg 2013;16:695-7. [Crossref] [PubMed]
- da Costa FD, Pereira EW, Barboza LE, et al. Ten-year experience with the Ross operation. Arq Bras Cardiol 2006;87:583-91. [PubMed]
- Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490-5. [Crossref] [PubMed]
- Sharabiani MT, Dorobantu DM, Mahani AS, et al. Aortic Valve Replacement and the Ross Operation in Children and Young Adults. J Am Coll Cardiol 2016;67:2858-70. [Crossref] [PubMed]
- Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967;2:956-8. [Crossref] [PubMed]
- Elkins RC, Thompson DM, Lane MM, et al. Ross operation: 16-year experience. J Thorac Cardiovasc Surg 2008;136:623-30, 630.e1-5.
- Brancaccio G, Polito A, Hoxha S, et al. The Ross procedure in patients aged less than 18 years: the midterm results. J Thorac Cardiovasc Surg 2014;147:383-8. [Crossref] [PubMed]
- Konno S, Imai Y, Iida Y, et al. A new method for prosthetic valve replacement in congenital aortic stenosis associated with hypoplasia of the aortic valve ring. J Thorac Cardiovasc Surg 1975;70:909-17. [PubMed]
- Ruzmetov M, Geiss DM, Shah JJ, et al. The Ross-Konno is a high-risk procedure when compared with the Ross operation in children. Ann Thorac Surg 2013;95:670-5. [Crossref] [PubMed]
- Mokhles MM, Rizopoulos D, Andrinopoulou ER, et al. Autograft and pulmonary allograft performance in the second post-operative decade after the Ross procedure: insights from the Rotterdam Prospective Cohort Study. Eur Heart J 2012;33:2213-24. [Crossref] [PubMed]
- Kato Y, Hattori K, Motoki M, et al. Optimal results of aortic valve replacement with small mechanical valves (< 19 mm). J Heart Valve Dis 2013;22:468-75. [PubMed]
- Talwar S, Malankar D, Garg S, et al. Aortic valve replacement with biological substitutes in children. Asian Cardiovasc Thorac Ann 2012;20:518-24. [Crossref] [PubMed]
- Turrentine MW, Ruzmetov M, Vijay P, et al. Biological versus mechanical aortic valve replacement in children. Ann Thorac Surg 2001;71:S356-60. [Crossref] [PubMed]
- Etnel JR, Elmont LC, Ertekin E, et al. Outcome after aortic valve replacement in children: A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2016;151:143-52.e1-3.