Current status and future perspectives of the PumpKIN trial
Introduction
There has recently been a continuous increase in the use of continuous-flow ventricular assist devices (VADs) in the pediatric population. Such an ongoing phenomenon is rapidly changing the outlook of pediatric heart failure management. With an increasing experience, however, it is also becoming apparent that the use of ‘adult’ VADs in ‘children’ is inherently problematic, primarily due to the patient-device size mismatch (1). Hence, the unmet need for the pediatric-specific continuous-flow VADs is being recognized.
This article describes the history, current status, and future perspectives of the special project supported by the National Heart, Lung, and Blood Institute (NHLBI), so-called PumpKIN (Pump for Kids, Infants, and Neonates) program. The specific goal of the project is to provide a solution to overcome the lack of circulatory support devices specifically for small children.
Robust use of continuous-flow VADs in children
According to the most recent report from the 2nd annual report of the PediMACS (pediatric counterpart of the INTERMACS registry: Interagency Registry for Mechanically Assisted Circulatory Support), 61% (174 out of 287) of durable VADs reported to the registry in the contemporary era are continuous-flow VADs (2). This phenomenon is primarily driven by the introduction of miniaturized continuous-flow VADs, such as the HVAD (Medtronic Inc., Minneapolis, MN, USA), into the pediatric practice. The worldwide survey of pediatric HVAD application recently conducted by Conway et al. has revealed that this phenomenon is not limited to North America (3). Out of the 205 patients included in this survey from 35 institutions and 12 countries, 123 (60%) were from North America while the remaining 82 (40%) were from European countries, Australia, Turkey, Israel, Japan and Egypt. Of note, implants occurred between 2009 and 2016, with the majority occurring within or after 2014 (67%). Certainly, the era of pediatric continuous-flow VAD support has begun.
With an increasing experience in pediatric continuous-flow VAD support, nonetheless, it is also becoming apparent that the benefits of continuous-flow VADs may not be uniform across all age groups within the pediatric population. In particular, there has been a concern regarding an elevated risk of device-related complications such as pump thrombosis in small children. The recent multi-institutional study (4) involving four pediatric centers focusing on continuous-flow VAD support in children with a BSA of <1.0 m2 reported a 31% (4 patients out of 13) incidence of pump thrombosis. The fundamental issue stems from the fact that a pump designed for adult hearts is used in small children (i.e., patient-device size mismatch). There is no question that the smaller the patient, the more significant mismatch exists. Despite the initial enthusiasm for the use of HVAD in small children (i.e., BSA 0.7 m2 or even smaller), clinicians have realized that the outcome of HVAD support in small children may not be as good as those with larger BSA. Thus, the unmet need for continuous-flow VADs specifically designed for the pediatric population, such as the Infant Jarvik 2015 (Jarvik Inc, New York, NY, USA), has been realized.
Update on the PumpKIN trial
The development of the Infant Jarvik 2015 may represent the breakthrough to this frustrating situation (5). This new pediatric-specific pump will be tested in the PumpKIN trial. This trial was originally designed as a prospective two-arm randomized study comparing the Infant Jarvik 2015 and the standard (and only) pediatric device, i.e., EXCOR (Berlin Heart, Inc. The Woodland, TX, USA). The study design, however, is now under re-evaluation, with a potential transition to a single-arm pivotal study, likely preceded by a single-arm feasibility trial. The discussion regarding study design modification is currently underway among the trial executive committee, participating centers, the NHLBI, and the U.S. Food and Drug Administration (FDA).
Developmental phase
As described in detail in the recent publication (6), the development pathway of pediatric continuous-flow VADs is an extremely long and tedious. Below is a summary of such a challenging task. The challenges faced various perspectives, which included engineering, financial, regulatory, academic, and clinical aspects. As a result of these complicated challenges, it has taken more than a decade to reach the point that the Infant Jarvik became ready to be tested in a clinical trial. It was in 2004 when the NHLBI launched the Pediatric Circulatory Support Program (7), which is the predecessor to the PumpKIN program. The Pediatric Circulatory Support Program supported the early developmental stage of five circulatory support systems for infant and children (3 continuous-flow VADs, 1 pulsatile VAD, and 1 ECMO).
Three of the five original devices including the Infant Jarvik, and additional one ECMO system (the Pediatric Cardiopulmonary Assist System: PediPL, University of Maryland), made to the next stage in 2010 under the PumpKIN program through a funding from the NHLBI (8). The objective of this funding was to receive the Investigational Device Exemptions (IDEs) from the U.S. FDA, which would require thorough in-vitro assessment as well as the pre-clinical assessment in the chronic animal model. None of the four devices under the PumpKIN program was able to achieve the IDE approval by the end of the contract in 2014. Additional funding was requested to complete the remaining work on the PumpKIN devices to obtain IDE approval. It was obvious, however, at that point that all the devices could not receive necessary funding due to the budget constraints resulting from the federal budget sequestrations starting in 2013. With such reality and other unmodifiable factors, the Infant Jarvik became the only remaining device that continued to be supported by the PumpKIN program.
The long journey continues. After all the necessary requirements were met, the IDE application for the Infant Jarvik 2000 (the predecessor of the Infant Jarvik 2015) was submitted to the FDA in April 2014. The application was disapproved. With new testing results and additional information and clarification, an amendment to the IDE application was submitted in September 2014. The application was again disapproved, with a specific concern for hemolysis. The mean Normalized Index of Hemolysis of the Infant Jarvik 2000 over the 5-hour in vitro testing using the bovine blood was nearly 10 times higher than the control device (BP-50, Medtronic). The level of hemolysis was not within the acceptable range for the clinical use.
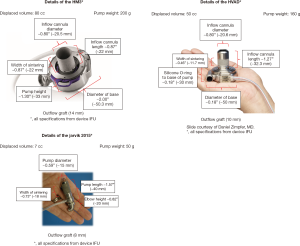
To address the hemolysis issue, various potential causes were considered, which included heating, cavitation, blade clearance, bearings, and material finishes. Between September 2014 and April 2015, multiple in vitro tests were performed at the Texas Heart Institute laboratory. These test results revealed that hemolysis was substantially reduced if the pump rotational speed was kept under 20,000 rpm, instead of the maximal 28,000 rpm. In order to keep the rpm below 20,000 while maintaining necessary pump flow, the decision was made to increase the pump outer diameter from 11 mm (Infant Jarvik 2000) to 15 mm (new Infant Jarvik 2015). The specifications of the Infant Jarvik 2015 and other adult continuous-flow VADs currently and/or potentially available for children are summarized in Figure 1. Once the new blade design was confirmed, in vitro hemolysis test was again repeated, which showed substantial reduction in hemolysis with the new pump (the mean Normalized Index of Hemolysis of the Infant Jarvik 2015 at the 3 litter per minutes flow condition showed nearly 10-fold reduction in hemolysis parameters compared to the old pump. It was felt that the new pump was ready for the pre-clinical assessment in a chronic animal model.
Pre-clinical phase
The detail of the pre-clinical assessment has been published elsewhere (5). The first task the team at the Texas Children’s Hospital faced was to establish “what to demonstrate in the chronic animal model” to satisfy the FDA. The answer to this seemingly obvious question was not so clear at the beginning of this project. There is no ‘standard’ way of testing pediatric VAD in the animal model. The prior in vivo chronic study with the old Infant Jarvik 2000 was conducted at a different institution. Due to the unpublished nature of the study, little information was available for us; in other words, we were unable to learn from what was successful and what was not. Although the Berlin Heart EXCOR received IDE approval from the FDA, that was primarily based on the already-existing clinical experiences, and not through the in-vivo animal data. In other words, the Infant Jarvik 2015 was the first pediatric VAD that tackled the thorough evaluation of the in vivo animal data by the FDA. The project team believed that to clarify what to demonstrate would determine how to design the study. The objective of the preclinical assessment, in a broad sense, was to test the feasibility of the new Infant Jarvik 2015 in a chronic animal model. More specifically, based on the concerns with the previous IDE application and the experience from the prior chronic animal study, the goals we established were: (I) healthy state of the animals over 2 months; (II) minimal hemolysis despite minimal anticoagulation; and (III) acceptable incidence of thromboembolic events. For these goals, we decided to use Barbados sheep weighing approximately between 20 to 30 kg. Although the intended weight range for clinical use is 20 kg or less, the hearts we treat in the real clinical setting are usually severely dilated. Thick, non-dilated hearts of healthy sheep of 20 to 30 kg range would represent a more challenging condition in terms of intra-ventricular cavitary size [i.e., the smaller and the more contractile, the more difficult for VAD support (5)]. The advantage of using Barbados sheep of this weight range is that this specific breed is already grown up and therefore there would be no much weight increase during the chronic state over 2 months. Somatic growth is a significant problem in a chronic ‘pediatric’ model; for example, if juvenile pigs are used for chronic study; weight may be more than double at the end of the study, making the validity of the study uncertain in terms of the target weight range. In the Infant Jarvik animal study, to demonstrate acceptable hemolysis profile, the pump speed was kept ‘as high as possible’ as the heart of the individual sheep tolerates. As neither imaging studies (e.g., echocardiography or X-rays) nor hemodynamic monitoring (e.g., blood pressure, etc.) were available during the chronic state, we defined the ‘tolerance’ of pump speed based on the changes in pump power consumption shown on the VAD controller, which was essentially the only information available to us. Anticoagulation also required extensive discussion; again there is no ‘standard’ way to anticoagulate these animals. To our mind, there were potentially two different options. One was to be on the aggressive side with anticoagulation in an attempt to protect the pump. The other was to be gentle with minimal anticoagulation so as to prove the pump would not cause hemolysis even without heavy anticoagulation. In the real clinical practice, it is not rare that patients on VAD support experience hemorrhagic complications, requiring temporary discontinuation of anticoagulation. We elected to take the latter approach in anticoagulation strategy.
As a result of clarifying the study goals and designing the study accordingly, the FDA granted IDE approval to the Jarvik Heart Inc. in September 2016. After 13 years of tedious pathway from the beginning of the Pediatric Circulatory Support program, the Jarvik pump finally reached a point where the device could be tested in human.
Clinical phase
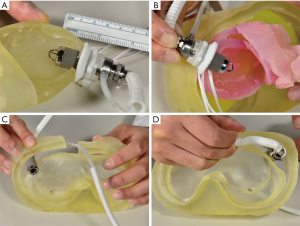
The development of a protocol for the clinical trial was an integral part of the IDE submission. Lengthy and multiple discussions occurred among the PumpKIN trial executive committee members (Study Chair: William Mahle, MD), involved consultants, NHLBI, manufacture, and clinicians at the participating vanguard sites. In particular, careful consideration was given to the weight range for the study participants. The final decision was to include children with a weight of 8 to 20 kg. The lower limit of 8 kg was chosen based on the expected flow range (approximately 1,200 mL per minutes =150 mL/kg ×8 kg). Because the pump speed was maintained as high as possible in the aforementioned chronic animal model, there would be no proof that the pump could maintain its functionality at a very low flow state (e.g., <1,000 mL per minutes). Virtual implantation was also conducted using the 3D print model of the heart and chest wall using computed tomography data from an 8 kg patient with dilated cardiomyopathy, confirming that the pump housing can be accommodated in the body of the 8 kg child if the pump is placed with some technical modifications (9) (Figure 2). The upper limit of 20 kg was chosen primarily based on the preliminary experience that children with 20 kg or larger could be supported with the commercially available device (i.e., HVAD) (10,11). The protocol was finalized and approved by the FDA. The plan was to initiate the trial with the vanguard sites (5 in the U.S. and 2 in Canada), followed by a subsequent expansion to 20+ centers in North America. By the fall in 2017, three vanguard sites including us became active in patient enrollment; the PumpKIN trial had finally begun. Although there were a couple of potential candidates identified in these activated sites, no patients were enrolled by the end of 2017. The PumpKIN executive committee decided to hold the trial in December 2017 to initiate the discussion to amend the study protocol. There is now an ongoing discussion involving the FDA regarding how the new trial design should be. Although it has not been finalized yet, the consensus opinion so far is that switching to the single-arm study (i.e., not comparing to the Berlin EXCOR) would be more appropriate.
As this is still an ongoing process, it would be too premature at this point to judge the original study design, i.e., a randomized controlled trial, which would probably be most ideal from the academic viewpoint. From a view of a clinician who discuss study enrollment with the patients’ family, the most significant hurdle of the original design was a randomization of two devices that would have very different risk-benefit profiles. An optimal timing for pulsatile and continuous-flow VAD implant is different; in general, the clinicians proceed with pulsatile VAD at INTERMACS profile 1 or 2 due to the risk profiles of the pulsatile VAD. Conversely, there is a general tendency toward earlier initiation (INTERMACS 3 or above) of continuous-flow VAD as the data clearly demonstrate that the outcome would be better if the VAD support is offered without delay. Potential for outpatient management is an advantage of the continuous-flow VAD support, while in-house management is currently mandatory with the pulsatile VAD. Due to the randomization and hence the possible assignment to the pulsatile VAD, the study protocol mandates the clinicians to manage all patients enrolled in the trial as if they had the pulsatile VAD (i.e., timing of implantation, the intensity of anticoagulation, and in-house care), which essentially spoil the benefit of being on a continuous-flow VAD. If the devices to be compared have similar risk-benefit profiles, a randomized control trial would be the most suitable study design. Excellent examples are the recent adult trials comparing the HVAD vs. HeartMate II (12) and the HeartMate 3 vs. HeartMate II (13). There are situations, nonetheless, where academic priority may be less prioritized given the practical reality of clinical practice. The optimal balance between being academic and practical is particularly difficult to find in the pediatric population. That would probably be one of the important lessons we have learned from this experience.
What would the PumpKIN bring?
We are hopeful that the pediatric field will have an access to the continuous-flow VAD specifically designed and approved for children at the conclusion of the PumpKIN trial. That would definitely accelerate the currently ongoing paradigm shift toward the use of continuous-flow VADs in the pediatric population (14). Gaining one additional pump, however, will not be the only achievement we would get from the entire experience of the PumpKIN program.
As discussed above, the lessons we have learned from the clinical study designing process should definitely help us in the preparation of future pediatric VAD trials. This was really a collaborative learning process involving not only clinicians but also the federal agencies (i.e., NHLBI and FDA).
We have also extensively learned that how to promote the device from a developmental/pre-clinical stage to a clinical trial phase by gaining an experience in the complex IDE application. The Infant Jarvik is certainly the first, and currently only, pediatric-specific VAD that underwent the thorough in-vitro and in-vivo assessment to satisfy the FDA requirement in the current era. The data submitted to the FDA for the Infant Jarvik 2015, which eventually resulted in approval of the IDE application, will serve as the benchmark for the future device development. In particular, the methodology utilized for the preclinical study using the chronic sheep model, which is the critical component of the FDA application, to test the feasibility of the Infant Jarvik 2015 will serve as the basis of the future device IDE application. The future devices can be tested in the nearly identical manner to the Infant Jarvik so the data obtained from the animal model would be comparable to that of the Infant Jarvik that reached the satisfaction of the FDA. Having the standard way to assess pediatric devices and the benchmark data would certainly facilitate the future device development and hopefully reduce the cost necessary for the complicated development process. To my mind, that would be the true, and most impactful, legacy of the entire PumpKIN program.
Summary
The history of the PumpKIN program (and its predecessor, namely the Pediatric Circulatory Support Program) has demonstrated the extremely challenging nature of pediatric-specific VAD development. However, as a collaborative effort among clinicians, scientists, manufactures, and federal agencies, there has been a steady progress, which is now coming to fruition as the initiation of a clinical trial to test the Infant Jarvik 2015, the first continuous-flow VAD specifically designed for small children. The lessons learned from this entire experience over the decade will surely have a substantial positive impact on the future device development for the pediatric population.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Adachi’s employer, Texas Children’s Hospital received a salary support for his role as a consultant with the New England Research Institute and the Sony-Olympus Medical solution, Inc. Dr. Adachi serves as a consultant/proctor for the Berlin Heart Inc. and HeartWare, Inc.
References
- Adachi I, Burki S, Fraser CD Jr. Current Status of Pediatric Ventricular Assist Device Support. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2017;20:2-8. [Crossref] [PubMed]
- Blume ED, VanderPluym C, Lorts A, et al. Second annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) report: Pre-implant characteristics and outcomes. J Heart Lung Transplant 2018;37:38-45. [Crossref] [PubMed]
- Conway J, Miera O, Henderson HT, et al. Global Experience with the Heartware HVAD® in Pediatric Patients: A Preliminary Analysis. J Heart Lung Transplant 2016;35:S45. [Crossref]
- Miera O, Kirk R, Buchholz H, et al. A multicenter study of the HeartWare ventricular assist device in small children. J Heart Lung Transplant 2016;35:679-81. [Crossref] [PubMed]
- Adachi I, Burki S, Horne D, et al. The miniaturized pediatric continuous-flow device: Preclinical assessment in the chronic sheep model. J Thorac Cardiovasc Surg 2017;154:291-300. [Crossref] [PubMed]
- Baldwin JT, Adachi I, Teal J, et al. Closing in on the PumpKIN Trial of the Jarvik 2015 Ventricular Assist Device. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2017;20:9-15. [Crossref] [PubMed]
- Baldwin JT, Borovetz HS, Duncan BW, et al. The National Heart, Lung, and Blood Institute Pediatric Circulatory Support Program. Circulation 2006;113:147-55. [Crossref] [PubMed]
- Baldwin JT, Borovetz HS, Duncan BW, et al. The national heart, lung, and blood institute pediatric circulatory support program: a summary of the 5-year experience. Circulation 2011;123:1233-40. [Crossref] [PubMed]
- Adachi I, Guzmán-Pruneda FA, Jeewa A, et al. A modified implantation technique of the HeartWare ventricular assist device for pediatric patients. J Heart Lung Transplant 2015;34:134-6. [Crossref] [PubMed]
- Peng E, Kirk R, Wrightson N, et al. An Extended Role of Continuous Flow Device in Pediatric Mechanical Circulatory Support. Ann Thorac Surg 2016;102:620-7. [Crossref] [PubMed]
- Nassar MS, Hasan A, Chila T, et al. Comparison of paracorporeal and continuous flow ventricular assist devices in children: preliminary results. Eur J Cardiothorac Surg 2017;51:709-14. [Crossref] [PubMed]
- Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure. N Engl J Med 2017;376:451-60. [Crossref] [PubMed]
- Mehra MR, Naka Y, Uriel N, et al. A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med 2017;376:440-50. [Crossref] [PubMed]
- Adachi I. Continuous-flow ventricular assist device support in children: A paradigm change. J Thorac Cardiovasc Surg 2017;154:1358-61. [Crossref] [PubMed]


