Is there any progress in the search for the ideal conduit for reconstruction of the right ventricular outflow tract in young children?
Obstructions of the right ventricular outflow tract (RVOT) are common pathologies in congenital heart disease. In the absence of an appropriate pulmonary valve, it is necessary to replace the RVOT with a valved conduit. But searching for the ideal conduit is an on-going task. The ideal conduit should have following characteristics: availability in any sizes, long-term durability, excellent hemodynamics, growth potential and be non-thrombogenic. Currently, such a conduit does not exist.
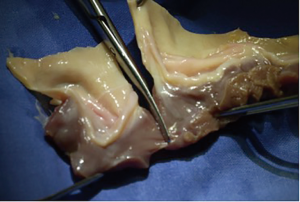
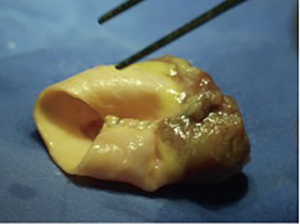
Over the years, surgeons have used a multitude of different conduits and compared them between each other (1,2). For decades, the usage of homografts was prevalent in congenital cardiac surgery with excellent long-term results. Several studies have demonstrated that homografts are durable and show excellent hemodynamics (3). Unfortunately, due to regulation changes in some countries and cultural particularities in others, homografts are not (any more) available in all countries of the world. But even if available, small-sized homografts are rare. This is the reason, why some surgeons started to “bicuspidalize” or “downsize” homografts, removing a cusp and sewing it back together to have a smaller-sized bicuspid homograft (Figures 1,2). This surgical method arose out of the need for small-sized homografts and is widely used in centres with access to homografts (4,5).
In their paper, François and colleagues report on 93 conduits sized 20 mm or less implanted in 88 patients (6). Endpoints of the study were survival, conduit replacement and structural valve degeneration (SVD). SVD was defined as a peak gradient of 40 mmHg or a pulmonary regurgitation >2/4 on echocardiography. Most implanted conduits were pulmonary homografts (n=40), followed by bicuspidalized homografts (n=17), aortic homografts (n=12) and bovine jugular vein conduits (Contegra®, n=24). Median patient age was 1.4 years (IQR: 0.3–3 years) and median weight was 8.8 kg (IQR: 5–12.9 kg). There were some distinctive differences between groups: patients with a pulmonary homograft were older compared to patients with a bicuspidalized homograft or a bovine jugular vein conduit. Furthermore, patients with an aortic homograft had the highest number of heterotopic implants. The median time to conduit exchange was not different between groups but ranged from 5.6 years for Contegra® to 8.3 years for aortic and bicuspidalized homografts. Looking at the freedom from SVD at 10 years, pulmonary homografts had the best results with 68%±8%, and Contegra® the worst with 20%±9% (P<0.001). The multivariate analysis showed that smaller conduit size (HR 0.79 per millimetre conduit size, 95% CI: 0.67–0.94, P<0.008), heterotopic implantation (HR 2.71, 95% CI: 1.33–5.5, P=0.006) and the use of Contegra® compared with pulmonary homografts (HR 4.9, 95% CI: 2.23–10.76, P<0.001) were significant risk factors for SVD. Risk factors for conduit exchange in the multivariate analysis were smaller conduit size (HR 0.69, 95% CI: 0.55–0.85, P<0.001) and the use of Contegra® compared with pulmonary homografts (HR 3.32, 95% CI: 1.48–7.42, P=0.004). Z-scores at implantation were higher for bicuspidalized homografts and Contegra® compared to pulmonary homografts (P=0.001). Z-scores at explantation were not different between groups and ranged from −0.8 for Contegra® to −2.1 for pulmonary homografts. The authors derived from these results, that the main reason for conduit exchange was outgrowth. Interestingly, endocarditis was only diagnosed in patients with a Contegra® (n=3, 13%).
The results of this study show again, that homografts have the best results regarding durability and hemodynamic properties. Smaller sized conduits are at risk of SVD and early exchange. These findings are not completely new and bring us back to the initial problem: what conduit should be chosen in neonates? Due to the substantial growth characteristics of neonates and the relatively small pulmonary arteries in contrast to any conduit, neonates are prone to early conduit failure. A neonate born at term, weighing 3.5 kg and measuring 52 cm would need a conduit of 9 mm. Because it is less probable to get a homograft of this size, most surgeons opt for larger diameters, as shown in the paper of Francois et al. The z-scores ranged between 2.2 and 3.5, which means that the patients received a conduit more than twice as large as required.
The other disillusioning finding is that bovine jugular vein conduits are not as good as homografts. In the literature, durability of bovine jugular vein conduits has been described differently. Some authors found comparable results to homografts (2,7), others not (8). These contradictory results can be explained by two aspects: different patient age and different end-points of the studies. Focussing on papers with small patients, Contegra® has shown recurrent problems such as dilatations and early failures (9,10).
Only few papers analysed conduit durability in neonates and infants in the first year of life (7). Due to the enormous growth potential of children in the first year of life, this is the patient population with the biggest challenge. In the paper of Francois et al., the patients were young but with a median age of 1.4 years, more than half of the patients were older than 1 year. Due to different methods including patients (age, weight, conduit size) in studies, results are somewhat difficult to compare. Although it remains clear, that young age and/or small conduits are the major risk factors for conduit exchange, the actual role of the type of conduit is not reliable and continues to be unknown. Until now, it is only known that the Contegra® develops earlier a moderate stenosis or moderate regurgitation. But the consequence does not necessarily result in a conduit exchange (7).
Most studies analyzing outcome of cardiac conduits in congenital heart surgery are focussing on durability. For a better comparability of studies, it is sensible to do so. But the time when to exchange a conduit remains controversial and is dependent on the attending physicians. In their study, Francois and colleagues chose SVD besides durability, as an end-point. Interestingly, the risk factors for both end-points were similar. In centres where patients are being followed up regularly, the difference between a conduit “failure” and a conduit “exchange” is probably quite similar due to early recognition and consecutive intervention.
Overall, the search for the ideal conduit is not at its end. We know that homografts are the best and that bicuspidalized homografts are a good option if small-sized homografts are not available. But in the setting of a lack of homografts, it remains unknown as to which commercial conduit to choose. The research is continuing to progress and some alternative conduits show promising results. Results of decellularized homografts show no explantation 10 years after implantation (11). These results are exceptional and should lead to a substantial change of management. Unfortunately, it doesn’t resolve the problem of homograft shortage. Some studies with fully synthetic bioabsorbable supramolecular polymers are starting with clinical trials (12). These studies will need to prove benefits over homografts in the long-term, which will take some years.
Hoping for the future is to hope for an individualized conduit, made of the patients’ own cells, lasting life-long and growing with the patient.
AcknowledgementsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The author has no conflicts of interest to declare.
ReferencesOther Section
- Dearani JA, Danielson GK, Puga FJ, et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg 2003;75:399-410; discussion 410-1. [Crossref] [PubMed]
- Boethig D, Thies WR, Hecker H, et al. Mid term course after pediatric right ventricular outflow tract reconstruction: a comparison of homografts, porcine xenografts and Contegras. Eur J Cardiothorac Surg 2005;27:58-66. [Crossref] [PubMed]
- Tweddell JS, Pelech AN, Frommelt PC, et al. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation 2000;102:III130-5. [Crossref] [PubMed]
- Cleuziou J, Vitanova K, Kasnar-Samprec J, et al. Durability of down-sized homografts for the reconstruction of the right ventricular outflow tract. Eur J Cardiothorac Surg 2016;49:1421-5. [Crossref] [PubMed]
- Perri G, Polito A, Gandolfo F, et al. Outcome of Standard and Bicuspidalized Cryopreserved Homografts for Primary Right Ventricular Outflow Tract Reconstruction. J Heart Valve Dis 2015;24:83-8. [PubMed]
- François K, De Groote K, Vandekerckhove K, et al. Small-sized conduits in the right ventricular outflow tract in young children: bicuspidalized homografts are a good alternative to standard conduits. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [PubMed]
- Vitanova K, Cleuziou J, Hörer J, et al. Which type of conduit to choose for right ventricular outflow tract reconstruction in patients below 1 year of age?. Eur J Cardiothorac Surg 2014;46:961-6; discussion 966. [Crossref] [PubMed]
- Urso S, Rega F, Meuris B, et al. The Contegra conduit in the right ventricular outflow tract is an independent risk factor for graft replacement. Eur J Cardiothorac Surg 2011;40:603-9. [PubMed]
- Meyns B, Van Garsse L, Boshoff D, et al. The Contegra conduit in the right ventricular outflow tract induces supravalvular stenosis. J Thorac Cardiovasc Surg 2004;128:834-40. [Crossref] [PubMed]
- Göber V, Berdat P, Pavlovic M, et al. Adverse mid-term outcome following RVOT reconstruction using the Contegra valved bovine jugular vein. Ann Thorac Surg 2005;79:625-31. [Crossref] [PubMed]
- Sarikouch S, Horke A, Tudorache I, et al. Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg 2016;50:281-90. [Crossref] [PubMed]
- Bennink G, Torii S, Brugmans M, et al. A novel restorative pulmonary valved conduit in a chronic sheep model: Mid-term hemodynamic function and histologic assessment. J Thorac Cardiovasc Surg 2018;155:2591-601.e3. [Crossref] [PubMed]