
Recommendation of plant-based diets for inflammatory bowel disease
Introduction
The incidence of inflammatory bowel disease (IBD), a collective term for Crohn’s disease (CD) and ulcerative colitis (UC), has been increasing over time and expanding to different regions around the world, indicating that IBD is a global disease. IBD is a polygenic disease thought to be triggered by environmental factors. IBD may represent dysregulated mucosal inflammation in response to gut microbiota. A Western or westernized lifestyle is thought to be a major driver of the growing incidence of IBD. Currently, however, the key environmental factor for IBD is unidentified.
It is imperative that we identify the ubiquitous environmental factor underlying IBD in order to provide suitable treatment. Findings from epidemiology studies have indicated that diets high in animal fat and low in fruits and vegetables are the most common pattern associated with an increased risk of IBD (1). We believe that westernized diet-associated gut dysbiosis is the most ubiquitous environmental factor in IBD (2). The next question then becomes what kind of diet is suitable for IBD as a replacement for a westernized diet. Due to a lack of evidence, the most suitable diet for IBD is unknown (1). This is a serious problem in current IBD practice. Patients are advised to eat a well-balanced diet, avoiding processed foods or foods that they self-identify as worsening their symptoms (1).
A chemically defined elemental diet is used for exclusive enteral nutrition. Exclusive enteral nutrition is recommended as a first-choice therapy in pediatric active CD in Europe. However, it has the disadvantage of a high discontinuation rate (1). In North America, it is used by fewer than 4% of pediatric gastroenterologists.
This commentary aims to provide a rationale for why we recommend a plant-based diet (PBD) for IBD. Here, we discuss regular diets.
Current pathogenesis of IBD
Recently, research has shown that diet shapes gut microbiota, which is related to our health and morbidity (3-5). We have coevolved with gut microbiota to exist in a symbiotic relationship. Westernized diets (high in fat, animal protein and sugar, low in dietary fiber) decrease Firmicutes and increase Bacteroidetes at the phylum level: Bacteroides dominate at the species level. In contrast, PBD (low in fat, animal protein and sugar, high in dietary fiber) induce largely opposite changes. They increase Firmicutes and decrease Bacteroidetes: Prevotella species dominate.
Overall, westernized diets tend to decrease microbial diversity (dysbiosis) and PBD tend to increase microbial diversity. This difference in microbiota results in differences in microbial metabolites. Westernized diets result in increased production of ammonia, indols, phenols, and sulphide that may be detrimental to our health. In addition, they result in decreased production of short-chain fatty acids like butyrate. These metabolites have diverse beneficial effects in nutrition, immunity, and epithelial barrier. PBD result in increased production of short-chain fatty acids.
Altogether, westernized diets are pro-inflammatory and PBD are anti-inflammatory (3-5). These observations indicate that westernized diets increase susceptibility to not only IBD but also other chronic diseases. The above phenomena associated with westernized diets are observed in IBD (6). Based upon the changes in the intestinal microbiota and metabolites, dietary intervention is justifiable in the treatment in IBD (1).
Criteria for suitable diet in IBD
The following four criteria may be helpful for designing a suitable diet in IBD.
Rationale for designing the diet
The rationale for designing the diet should be based on the pathogenesis of IBD. Currently, dysbiosis of gut microbiota is found not only in IBD but also in other chronic diseases. The suitable diet will restore symbiosis of gut microbiota. Because UC and CD have similarities as well as differences in pathogenesis, phenotype, response to medication and so on, there might be some difference in the suitable diet between UC and CD.
Efficacy of the diet in IBD
IBD has two stages: active and quiescent stage. The treatment goal is induction of remission in the active stage and maintenance of remission in the quiescent stage. The epidemiology of IBD is similar in both CD and UC. This means that the suitable diet should be effective in both UC and CD. If the diet is suitable, the diet can be provided from the start of treatment even in the active stage with or without medication. The exception is the fulminant stage, when patients lose their appetite and do not want to eat. The induction rate and maintenance rate of remission with/without medication should be higher with the suitable diet than with a conventional diet.
Acceptability of the diet by patients
The suitable diet should be accepted by the majority of IBD patients. The patient or meal preparer should be able to prepare the diet, and patients should be able to eat the diet. For this purpose, the naming of the diet is important. The name of the diet should enable patients to easily imagine the content of the diet. For example, it would be difficult for the average person to imagine the content of a low fermentable oligo, di, monosaccharide and polymer (Low-FODMAP) diet.
World-wide application of the diet
IBD is a global disease. Therefore, the diet should be applicable worldwide.
In the literature, there are primitive reports of several diets for IBD: the specific carbohydrate diet, low-FODMAP diet, IgG4-guided exclusion diet, autoimmune protocol diet, an anti-inflammatory diet, and PBD (1). They are far from satisfying the above criteria, except for PBD. PBD is a plausible candidate for the suitable diet for IBD.
History and concept of PBD in IBD
History
The term PBD includes diets of various moderating degrees of animal foods ranging from a strict vegan diet to a lacto-ovo-vegetarian diet. We recognized IBD as lifestyle disease mediated mainly by westernized diets (7). Westernized diets tend to decrease beneficial gut microbiota. We expected vegetables to increase beneficial bacteria. Considering the acceptability of diets by patients, a loose vegetarian diet, i.e., a lacto-obo-vegetarian diet with fish once a week and meat once every 2 weeks, was developed. We named it semi-vegetarian diet (7). The semi-vegetarian diet has been provided to all of our IBD patients since 2003. It appeared for the first time in the literature in 2005 in a case report (8), and in 2010 in an original paper (7). We began to use PBD rather than semi-vegetarian diet since 2016 because PBD seemed to be more familiar to the public than vegetarian diet (9).
Concept of PBD
It is natural that preventable diets for disease are recommended and risky diets are moderated. There will be a limitation to both mere addition of probiotics, prebiotics or foodstuffs and mere exclusion of potentially untoward food stuffs (1,7). We believe that not partial but comprehensive food control is needed for patients who are genetically predisposed to IBD. PBDs are listed as variations of USDA healthy eating patterns and recommended to the public to prevent common chronic diseases (10,11). PBDs are appropriate for all stages of the life cycle, including infancy, childhood, and adolescence (11).
Our PBD was described in detail previously (7). Fruits, vegetables, legumes, potatoes, and yoghurt are served daily. Sweets are not served. We developed a PBD scoring system for Japanese patients with IBD (9). The plant-based diet score (PBDS) evaluates adherence to PBD. Eight items considered to be preventive factors for IBD (vegetables, fruits, pulses, potatoes, rice, miso soup, green tea, and plain yogurt) contribute to a positive score (PBDS+), while eight items considered to be IBD risk factors (meat, minced or processed meat, cheese/butter/margarine, sweets, soft drinks, alcohol, bread, and fish) contribute to a negative score (PBDS−). Scores of 5, 3, and 1 are given according to the frequency of consumption: every day, 3–5 times/week, and 1–2 times/week, respectively. The PBDS is calculated as the sum of the positive and negative scores, and it ranges between −40 to +40. A higher PBDS indicates greater adherence to the PBD (9). The PBD and PBDS can be modified for a variety of diseases and for different national dietary preferences (9). No food item was prohibited (7).
PBD normalize bowel movements in constipated patients and induce normal stool in patients with diarrhea/loose stool. Normal bowel habit may indicate symbiosis of gut microbiota. This symbiosis will suppress onset of relapse. It is much like precautions against fire: suppression of ignition (Figure 1).
Our loose PBD, a lacto-ovo-semi-vegetarian diet allowing fish once a week and meat every other week, does not result in micronutrient deficiency. Those following a strict PBD, i.e., vegans, however, must take adequate vitamin B12 and vitamin D supplements, and must ensure proper calcium and iron intake (11).
Induction and maintenance of remission by therapy incorporating PBD
We have 15 years of experience treating more than 159 UC patients and 70 CD patients (9). This includes 15 UC patients from 11 to 18 years old and 16 CD patients from 13 to 18 years old (9). Our cases, even mild cases, were treated on an inpatient basis (12). In most of the patients, there was no hesitation about admission because they were aware of the abnormality and wanted to be treated. PBD was served after admission.
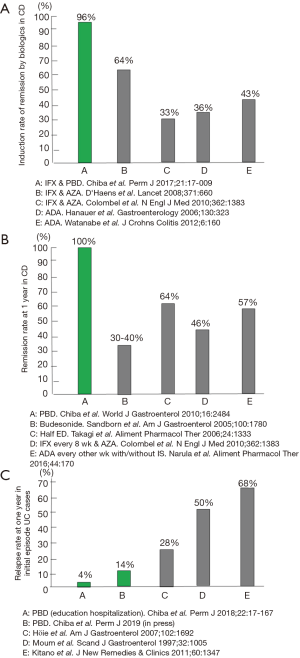
In CD, infliximab combined with PBD induced remission at a rate of 96% in 46 consecutive cases (13). This remission rate is excellent considering that around 30% of sufferers are primary non-responders to infliximab (14) (Figure 2A). PBD is effective in the maintenance of remission in CD: 100% and 90% at 1- and 2-year follow-up, respectively (7) (Figure 2B). This remission rate was achieved without scheduled maintenance therapy of infliximab or adalimumab. The relapse rate of initial episode patients with UC who were treated with therapy incorporating PBD was 14% at 1 year (15). It was 4% for educationally hospitalized patients (12) (Figure 2C). We now treat mild cases of UC with PBD first, not medication (12,15-17). PBD without medication can induce remission in about one-third of patients with mild UC (15). All patients ate the PBD, and none experienced an adverse effect (7,15). The rate of adherence to PBD during follow-up was 73% (7). Significant adherence to PBD determined by PBDS relative to baseline was maintained even 6 years later (15). Although admitting that therapeutic modalities are different, to our knowledge, these results are the best among those reported in the literature (Figure 2).
We believe the incorporation of PBD in therapy contributed most to our achievement. In our practice, hospitalization plays a critical role in replacement of an omnivorous diet with PBD. It is obvious that the majority of diseases we face are chronic diseases (lifestyle diseases) due to an unhealthy lifestyle (18). Therefore, incorporation of a healthy lifestyle in medicine, namely lifestyle medicine, is fundamental for prevention and treatment of chronic diseases. Changes in lifestyle including dietary habits are not easy. Hospitalization helps limit risk factors for IBD and our health such as smoking, alcohol, sweets, and animal foods, while patients benefit from preventive factors every day such as intake of vegetables and fruits (14,15). Hospitalization seemed to enhance their self-management skills, which contributed greatly to prevention of relapse (12,15).
Although some experts recommend avoidance of dietary fiber in the active phase, our experience refutes this. We provided PBD (containing a high amount of dietary fiber) in the active phase without any problems, even in patients with the stricture form of CD (7,12,13,15,19,20).
Limitation of PBD in IBD
The precise mechanisms underlying how diet induces microbial dysbiosis and results in onset of individual chronic diseases are not yet fully understood. Gut microbiota is constituted of not only bacteria but also viruses and fungi. New critical information relating to the pathogenesis of IBD might emerge from future investigations of microbiota rather than bacteria.
The evidence level of our clinical observations without a control diet is low as compared with a conventional randomized controlled trial. Larger controlled studies are needed to validate our results. Considering the difficulty of such a dietary intervention trial, however, it might take another decade to obtain convincing evidence on a suitable diet for IBD.
Conclusions
Based on the rationale of the diet, gut microbiota, and health consequences and excellent outcomes with therapy incorporating PBD, PBD is unreservedly recommended for IBD.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lewis JD, Abreu MT. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017;152:398-414.e6. [Crossref] [PubMed]
- Chiba M, Nakane K, Komatsu M. Westernized diet is the most ubiquitous environmental factor in inflammatory bowel disease. Perm J 2019;23. [PubMed]
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559-63. [Crossref] [PubMed]
- Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 2014;20:779-86. [Crossref] [PubMed]
- Simpson HL, Campbell BJ. Review article: dietary fiber-microbiota interactions. Aliment Pharmacol Ther 2015;42:158-79. [Crossref] [PubMed]
- Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11:1-10. [Crossref] [PubMed]
- Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn's disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol 2010;16:2484-95. [Crossref] [PubMed]
- Chiba M, Akashi T, Ando H, et al. Three Japanese cases of inflammatory bowel disease associated with left-sided colonic diverticulosis: Its implication. Inflamm Bowel Dis 2005;11:952-4. [Crossref] [PubMed]
- Chiba M, Nakane K, Takayama Y, et al. Development and application of a plant-based diet scoring system for Japanese patients with inflammatory bowel disease. Perm J 2016;20:62-8. [PubMed]
- The 2015 Dietary Guidelines for Americans. Washington, DC: US Department of Health and Human Services, 2015.
- Grant JD. Time for change Benefits of a plant-based diet. Can Fam Physician 2017;63:744-6. [PubMed]
- Chiba M, Nakane K, Tsuji T, et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: A single-group trial. Perm J 2018;22:17-167. [PubMed]
- Chiba M, Tsuji T, Nakane K, et al. Induction with infliximab and plant-based diet as first-line (IPF) therapy for Crohn disease: A single-group trial. Perm J 2019;23:18-107. [PubMed]
- Chiba M, Tsuji T, Nakane K, et al. How to avoid primary nonresponders to infliximab in Crohn’s disease. Inflamm Bowel Dis 2017;23:E55-6. [Crossref] [PubMed]
- Chiba M, Nakane K, Tsuji T, et al. Relapse prevention by incorporative plant-based diet in induction phase for ulcerative colitis: A single-group trial. Perm J 2019. In press.
- Chiba M, Tsuda S, Komatsu M, et al. Onset of ulcerative colitis during low-carbohydrate weight-loss diet and its treatment with plant-based diet: a case report. Perm J 2016;20:80-4. [PubMed]
- Chiba M, Sugawara T, Komatsu M, et al. Onset of ulcerative colitis in the second trimester after emesis gravidarum: treatment with plant-based diet. Inflamm Bowel Dis 2018;24:e8-9. [Crossref] [PubMed]
- Chiba M, Nakane K, Komatsu M. Lifestyle medicine in inflammatory bowel disease. Perm J 2018;22:18-062. [PubMed]
- Chiba M, Ohno H, Ishii H, et al. Plant-based diet in Crohn’s disease. Perm J 2014;18:94. [PubMed]
- Chiba M, Tsuji T, Nakane K, et al. High amount of dietary fiber not harmful but favorable for Crohn disease. Perm J 2015;19:58-61. [Crossref] [PubMed]


