
Ferric carboxymaltose in the treatment of iron deficiency in pediatric inflammatory bowel disease
Introduction
Iron deficiency (ID) with or without anemia is one of the most frequent complications of inflammatory bowel disease (IBD), with paediatric studies demonstrating a prevalence of more than 70% (1-3), with children more often affected than adults (1). Amongst patients with IBD, ID has substantiative impacts on quality of life (4), with additional impairments in cognitive function (5-7), fatigue and physical performance (8). The consequences of ID are potentially more important in children as ID has a detrimental impact on the developing brain (9-11) as well as impacting schooling and social development. Given the consequences of ID, it is important to correct depleted stores effectively. Repletion of iron stores results in improvement in quality of life measures that are unrelated to IBD disease activity (12). Despite this, ID remains consistently undertreated, despite a number of available therapeutic preparations.
Intravenous iron replacement therapy is the recommended method of administration in adults for moderate to severe anemia (13), but this mode of administration is less often employed in children, likely for a number of reasons, including a general reluctance to employ repeated intravenous infusions for children, as well as a paucity of safety and efficacy data. Oral iron supplements are often initiated in patients with ID without anemia, or with mild anemia, and are often considered first line therapy. Oral iron is a low cost and non-invasive method of correction. However oral iron is poorly tolerated, ineffectively absorbed, and can take 2–3 weeks to begin to increase hemoglobin (Hb) concentrations, and up to 6 months to correct iron stores (14). In addition, clinical studies have demonstrated that oral iron impacts the gut microbiome and promotes intestinal inflammation in active disease (15-17).
Many studies have shown efficacy and safety with iron sucrose as intravenous iron therapy (18-20), and is a commonly utilised preparation in paediatrics in North America. However, concerns exist over iron uncomplexing with sucrose at high dose, and therefore dosing is limited. Multiple infusions are usually required to replenish iron stores, which increases direct health care costs and burden on the patient.
Ferric carboxymaltose (FCM) is an intravenous iron preparation that can be administered in single doses up to 1,000 mg of iron in 15–20 min. This study aims to demonstrate the efficacy and safety of FCM in the pediatric IBD population, as well as determine the utility of practical administration in an observational cohort study.
Methods
Study participants
We conducted a single-center uncontrolled prospective cohort study of children with IBD treated with FCM between April 2012 and February 2014. Since 2012 the Queensland Children’s Hospital in Brisbane, Australia has used FCM for the treatment of ID in IBD and other chronic inflammatory conditions. Originally used in patients 14 years of age and over, encouraging pilot efficacy and safety data in this population prompted this prospective non-interventional study in patients 6 years of age and over with IBD. Written informed consent was obtained for all participants. The study was approved by the ethics committee in Queensland.
Patients with IBD aged 6 years and over with documented ID were eligible for treatment with FCM. ID in subjects without biochemical evidence of inflammation was defined as serum ferritin <30 µg/L or transferrin saturation (TS) <16%; In subjects with biochemical evidence of inflammation, ID was defined as ferritin <100 µg/L or TS <16%. Potential subjects were excluded if they had received a blood transfusion in the preceding 4 weeks. No study subject had coexisting B12 deficiency.
Demographic data were recorded for each participant, and included age at enrolment, gender, and diagnosis. No patient received oral iron or other preparation of intravenous iron throughout the study period.
Interpretation of lab values
Anemia in patients with IBD was defined by general World Health Organization (WHO) criteria, (Hb <115 g/L for patients between 5–11 years of age, <130 g/L in males 12 years and older and <120 g/L in females 12 years and older) regardless of disease activity. Evidence of biochemical inflammation was defined as C-reactive protein (CRP) >5. Prior to FCM administration biochemical parameters measured were: Hb, mean corpuscular volume (MCV), ferritin, and TS. CRP was measured as a marker of co-existing inflammation, with a value of >5 mg/L considered abnormal.
FCM infusion
Patients received at least one dose of FCM at 15 mg/kg (up to 1,000 mg) over 15–20 min. Nursing staff were present during the infusion and for 1 hour following, and made frequent assessments of pulse, blood pressure, temperature, and oxygen saturations. Adverse drug reactions were recorded by the nursing staff and treating physicians, where they were documented in the patient notes. Once patients were discharged from hospital, nursing or medical staff were available by phone for reporting of adverse events. Follow- up laboratory testing was performed to assess efficacy, at approximately 8 weeks post infusion. FCM infusions were often performed opportunistically during routine visits to the hospital (e.g., at the time of endoscopic assessment, biologic infusions, clinic visits). A second infusion was offered to patients with incomplete resolution of ID in some circumstances, considering patient availability and the clinical context, as determined by their treating physician.
Statistical analysis
Descriptive statistics were reported as medians with interquartile range (IQR). Differences in demographic and laboratory parameters before and after FCM administration were determined using a Wilcoxon signed rank test. The effect of disease activity on response to FCM was determined by categorically grouping patients with normal and abnormal CRP and testing for statistical differences in response using a Pearson chi-squared test, and reported as odds ratios (OR) with 95% confidence interval (CI) based on the raw 2×2 tables. The effect of diagnosis (UC vs. CD) was also examined using a Pearson chi-squared test. A significance level of 0.05 was used throughout. All statistical analyses were performed utilizing IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Patients
In total, 139 patients with IBD underwent treatment with FCM between April 2012 and February 2014. Of these, 101 had follow-up iron studies (73%) and were included in the analysis. All patients who received treatment with FCM were included in the safety analysis. Sixty-four (63%) of patients were male, with a median age of 14 years (IQR 14–16). The youngest patient enrolled was 6 years old. Seventy-five (74%) of patients had CD, with 26 (26%) having UC. Fifty-seven (56%) of patients had persistently active disease throughout their study period, as defined by a consistently abnormal CRP.
A total of 44% of infusions were administered for patients with iron deficiency anemia (IDA), with a median Hb 111 (IQR, 105–116), and median TS 8 (IQR, 6–11). For patients with ID (56%), median TS was 11 (IQR, 9–14). Follow-up laboratory testing was available for all patients at a median time-frame of 8 (IQR, 6–8) weeks. The patient characteristics are noted in Table 1.
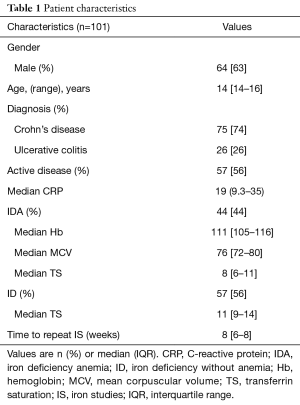
Full table
IDA
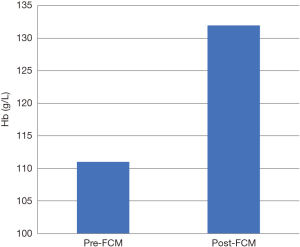
For patients who underwent infusion with FCM for IDA, 28 of 44 (64%) of patients showed resolution of anemia: 23 of 28 required one infusion, 5 required two infusions. Median Hb increased from 111 (IQR, 105–116) to 132 (120–139) (P<0.001), shown in Figure 1, and median TS increased from 8 (IQR, 6–11) to 20 (IQR, 14–29) (P<0.001).
ID without anemia
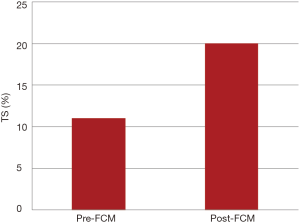
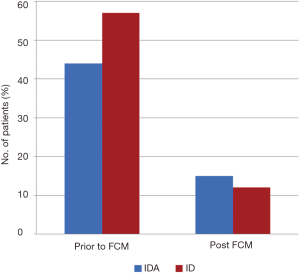
In patients who underwent FCM infusion for ID without anemia, 46 of 57 (81%) showed resolution of ID: 43 required one infusion, three required two infusions. The median TS increased from 11 (IQR, 9–14) to 20 (IQR, 16–29), shown in Figure 2. The numbers of patients with IDA and ID before and after administration of FCM are represented graphically in Figure 3.
Response by disease type
Of the 75 patients with Crohn’s disease, 32 (43%) had IDA. Of these, 22 (68.7%) had resolution of anemia following treatment with FCM. Of the 26 patients with Ulcerative Colitis, 12 (46%) had IDA, with 8 (67%) having resolution following treatment. There was no statistical difference between these groups (P=0.76).
Response by disease activity
In patients with active disease for the duration of the study period, 16 of 27 (59.3%) patients with IDA had resolution of anemia, while 10 of 16 (62.5%) of patients with quiescent disease had resolution of anemia. This was statistically non-significant (P=0.68).
However, in patients with ID without anemia, 22 of 31 (71%) of patients with active disease had resolution of ID, but 25 of 27 (93%) of patients with quiescent disease had resolution of ID (Chi-square, P<0.001, OR 5.1; 95% CI: 1.1 to 26, P=0.03).
Adverse events
Two patients had adverse reactions during our study period. One was a 14-year-old male, who experienced itch, urticarial rash over his trunk and legs, with a low-grade fever during the first 5 minutes of the infusion. This resolved with discontinuation of the infusion and administration of oral antihistamine (Cetirizine, 5 mg/dose). The patient refused further treatment with FCM. The second patient was an 8-year-old female, who also experienced low grade fever, urticarial rash and itch. This also resolved with discontinuation of the infusion, and administration of antihistamine (Cetrizine, 5 mg/dose). This patient received a further dose of FCM at a later date, with premedication (Cetirizine, 5 mg/dose, Paracetamol 15 mg/kg/dose), without a subsequent adverse event. Ten patients complained of pain around the infusion site. These all resolved by reducing the rate of administration by 50% and applying a warm compress to the area. There were no serious adverse events seen throughout the study period.
Discussion
Despite convincing evidence that ID causes significant morbidity in children with IBD, it remains prevalent and undertreated. Part of the issue relates to the difficulty in accessing appropriate intravenous therapies due to universal strain on resources. The efficacy and tolerability of FCM has been shown in various indications including IBD (21,22), and when compared to iron sucrose in patients with IBD was found to be more efficacious in achieving Hb response (22). A recent systematic review and meta-analysis demonstrated that FCM was the most effective treatment for IDA in IBD (23), with few studies in pediatrics with a proportion of patients with IBD demonstrating encouraging safety and efficacy (24,25).
This study demonstrated the practicality and efficacy of FCM in the treatment of ID, without the need for frequent infusions or long-term oral therapy. In our cohort of patients treated with FCM, 52% of patients with IDA demonstrated correction after one infusion, and 75% of patients with ID experienced correction with one infusion. All eight of the patients who received two FCM infusions for the instance of ID (five with IDA, three with ID) experienced normalization of laboratory markers.
In addition, we demonstrated the safety profile of FCM in pediatric patients, where evidence is limited. Our study noted two patients with adverse events characterised by mild urticarial rash, itch and low-grade fever, both of which resolved with administration of oral antihistamine, and no severe reactions or anaphylaxis. This is consistent with adverse events noted in the literature (21).
Oral iron remains frequently employed in patients with IBD for ID as first line therapy, especially when anemia is mild or in quiescent disease. With FCM, rapid correction of ID can be achieved with a single dose, with minimal strain on resources and few side effects. We feel therefore, that this should be considered as a potential first line therapy for all patients with ID. There are data in the adult literature demonstrating that correction of ID with FCM improves fatigue in a single dose (26). This would potentially have a similar effect in children, and so the ability to make a therapeutic difference with a single, rapid infusion should be considered.
This study additionally assesses the impact of disease activity on FCM effectiveness. It could be hypothesised that improvement in iron status is related to improvement in overall disease burden, as iron absorption blockade is reduced, bleeding subsides and malnutrition improves. In our cohort of patients, there was no statistical difference in treatment response in anaemic patients between those with persistent active disease (defined by CRP >5) and quiescent disease. However, in patients with only ID, there was a statistical difference in likelihood of resolution in patients with active vs. quiescent disease. Although 62% of patients still had resolution of anemia despite persistent activity, FCM almost universally corrected ID in those with quiescent disease. This demonstrates that despite having co-existing anemia of chronic disease where iron replacement is of no benefit, many patients will still benefit from treatment with iron during active disease.
There are some limitations in this study that should be noted. Due to the pragmatic study design, no specific iron deficit was calculated, and therefore iron requirements for the patients may have been underestimated. This was done purposely to help clarify the effectiveness of standard dosing of FCM, which is the most practical approach. Another limitation is that no attempt was made with our data collection to clarify the impact on iron stores of different IBD therapies. However, our observation that persistent biochemical evidence of disease activity had limited impact on iron infusion effects (especially in anaemic patients) points to the fact that FCM is effective regardless of treatment strategy. In addition, whilst exploration of this was outside the scope of this study, a large proportion of patients underwent treatment with FCM at the time of treatment with infliximab. The use of FCM in this setting only adds a short additional period of time for the infusion, limiting additional strain on resources, and is convenient for patients. A further limitation of our study is that there is no comparison group, either placebo or oral iron, and we perhaps used intravenous therapy when oral supplementation could have sufficed. We would argue that single dose FCM is a simpler, more practical way to achieve iron sufficiency.
Given our short period of follow-up for patients, we have no long-term data examining the durability of iron treatment with FCM. Anemia in IBD frequently relapses, especially in the face of active disease. In our patient population, several patients had more than one infusion of FCM during the study period, indicating that despite effective correction, ID reoccurs, especially in the face of active disease. This again shows the benefit of FCM as a preferred iron treatment, given the rapid infusion time and high dosing availability.
Conclusions
This study demonstrates the safety and efficacy of FCM in the treatment of ID in pediatric patients with IBD. Given the rapid infusion time and high dosing availability, it is a practical and effective way to reduce the burden of ID in these patients, and allows for repeated infusions without overt straining on resources.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Children’s Health Queensland Human Ethics Research Committee (EC00175) and informed consent was taken from all patients.
References
- Goodhand JR, Kamperidis N, Rao A, et al. Prevalence and management of anemia in children, adolescents, and adults with inflammatory bowel disease. Inflamm Bowel Dis 2012;18:513-9. [Crossref] [PubMed]
- Carvalho FSG, de Medeiros IA, Antunes H. Prevalence of iron deficiency anemia and iron deficiency in a pediatric population with inflammatory bowel disease. Scand J Gastroenterol 2017;52:1099-103. [Crossref] [PubMed]
- Gerasimidis K, Barclay A, Papangelou A, et al. The epidemiology of anemia in pediatric inflammatory bowel disease: prevalence and associated factors at diagnosis and follow-up and the impact of exclusive enteral nutrition. Inflamm Bowel Dis 2013;19:2411-22. [Crossref] [PubMed]
- Wells CW, Lewis S, Barton JR, et al. Effects of changes in hemoglobin level on quality of life and cognitive function in inflammatory bowel disease patients. Inflamm Bowel Dis 2006;12:123-30. [Crossref] [PubMed]
- Agaoglu L, Torun O, Unuvar E, et al. Effects of iron deficiency anemia on cognitive function in children. Arzneimittelforschung 2007;57:426-30. [PubMed]
- Jáuregui-Lobera I. Iron deficiency and cognitive functions. Neuropsychiatr Dis Treat 2014;10:2087-95. [Crossref] [PubMed]
- Sachdev H, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr 2005;8:117-32. [Crossref] [PubMed]
- DellaValle DM. Iron supplementation for female athletes: effects on iron status and performance outcomes. Curr Sports Med Rep 2013;12:234-9. [Crossref] [PubMed]
- Lukowski AF, Koss M, Burden MJ, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci 2010;13:54-70. [Crossref] [PubMed]
- Fretham SJ, Carlson ES, Wobken J, et al. Temporal manipulation of transferrin-receptor-1-dependent iron uptake identifies a sensitive period in mouse hippocampal neurodevelopment. Hippocampus 2012;22:1691-702. [Crossref] [PubMed]
- McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr 2007;85:931-45. [Crossref] [PubMed]
- Pizzi LT, Weston CM, Goldfarb NI, et al. Impact of chronic conditions on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis 2006;12:47-52. [Crossref] [PubMed]
- Reinisch W, Staun M, Bhandari S, et al. State of the iron: how to diagnose and efficiently treat iron deficiency anemia in inflammatory bowel disease. J Crohns Colitis 2013;7:429-40. [Crossref] [PubMed]
- Camaschella C. Iron-deficiency anemia. N Engl J Med 2015;372:1832-43. [Crossref] [PubMed]
- Kortman GA, Raffatellu M, Swinkels DW, et al. Nutritional iron turned inside out: intestinal stress from a gut microbial perspective. FEMS Microbiol Rev 2014;38:1202-34. [Crossref] [PubMed]
- Mahalhal A, Williams JM, Johnson S, et al. Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS One 2018;13:e0202460. [Crossref] [PubMed]
- Lee T, Clavel T, Smirnov K, et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017;66:863-71. [Crossref] [PubMed]
- Stein J, Hartmann F, Dignass AU. Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol 2010;7:599-610. [Crossref] [PubMed]
- Stein RE, Plantz K, Maxwell EC, et al. Intravenous Iron Sucrose for Treatment of Iron Deficiency Anemia in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 2018;66:e51-5. [Crossref] [PubMed]
- Schröder O, Mickisch O, Seidler U, et al. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol 2005;100:2503-9. [Crossref] [PubMed]
- Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol 2008;103:1182-92. [Crossref] [PubMed]
- Evstatiev R, Marteau P, Iqbal T, et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011;141:846-53.e1-2.
- Aksan A, Isik H, Radeke HH, et al. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017;45:1303-18. [Crossref] [PubMed]
- Laass MW, Straub S, Chainey S, et al. Effectiveness and safety of ferric carboxymaltose treatment in children and adolescents with inflammatory bowel disease and other gastrointestinal diseases. BMC Gastroenterol 2014;14:184. [Crossref] [PubMed]
- Tan ML, Windscheif PM, Thornton G, et al. Retrospective review of effectiveness and safety of intravenous ferric carboxymaltose given to children with iron deficiency anaemia in one UK tertiary centre. Eur J Pediatr 2017;176:1419-23. [Crossref] [PubMed]
- Stein J, Aksan A, Klemm W, et al. Safety and Efficacy of Ferric Carboxymaltose in the Treatment of Iron Deficiency Anaemia in Patients with Inflammatory Bowel Disease, in Routine Daily Practice. J Crohns Colitis 2018;12:826-34. [Crossref] [PubMed]


