
Predicting and preventing complications in children with inflammatory bowel disease
Introduction
Crohn’s disease (CD) and ulcerative colitis (UC) are two major forms of immune mediated chronic progressive inflammatory bowel disorders (IBD). A widely accepted hypothetical model of IBD pathophysiology suggests it is caused by an environment trigger in a genetically predisposed individual leading to mucosal epithelial barrier dysfunction, aberrant gut microbiota and immune dysfunction (1). Epidemiological trends suggest IBD is an emerging global disease, with rising prevalence in newly industrialised nations. A recent systematic review suggests the incidence of IBD in newly industrialised nations such as Asia, Africa and South America is increasing while the prevalence remains static in Europe and North America (2).
Although 10% of IBD is diagnosed before 16 years of age, the clinical course of a paediatric onset IBD is characterised by greater disease burden compared to adult-onset IBD (3,4). There are additional challenges of this age group, including growth, puberty and psychosocial consequences in such a vulnerable group and a greater risk of gastrointestinal (GI) cancers compared to non-IBD paediatric population. Cohort studies comparing the clinical course of paediatric-onset CD (PO-CD) vs. adult-onset CD (AO-CD) confirm its aggressive nature with extensive distribution, suggested by greater pan-enteric involvement [ileocolonic and upper gastrointestinal (GI) disease], rapid progression and increased disease activity index, year by year, despite use of more immunosuppression (5,6). In addition to higher disease burden, a greater cumulative risk of intestinal resection at 30 years (48% vs. 14%) and permanent stoma (12% vs. 1%) are seen in PO-CD vs. AO-CD (7).
UC in children also exhibits a more severe intensity in comparison to adults with UC. Children are more likely to present with extensive colitis, develop acute severe colitis, show a poor response to corticosteroids (CS) and up to 40% require colectomy within 10 years of diagnosis compared to 20% of adult onset UC (6,8-10). Population-based paediatric cohort studies suggest that 26–50% of children with UC may become CS dependent (steroid dependent), within the first year of diagnosis, compared to 22% of adults (10-12).
Despite a clear recognition that Paediatric-onset IBD is severe in comparison to an adult-onset IBD, not all children will have aggressive disease course. It is therefore essential to accurately predict those who are likely to develop a complicating disease course. Disease course of IBD is closely linked with treatment targets and choice of therapies. Declining 5-year surgical resection rates in CD, from 59% [1986–1991] to 25% [1998–2003] in UK and 30% [1988–1995] to 18% [2001–2008] in Canada may reflect the expanding armamentarium of treatment choices and improvement in treatment paradigms (13,14).
Paediatric studies report 5-year surgical resection ranges between 14–34% (15-17). Some recent studies report no change in surgical resection rates in childhood onset CD, despite increase utilisation of biologics (17). Although colectomy rates of hospitalised UC patients have also remained stable over years, more recent adult data confirm diminishing 10-year surgical resection rates from 30% in the 1990’s to 10–17% in recent studies (18). Colectomy rates are higher in paediatric-onset UC compared to adult UC, with population-based registries reporting 10-year risk around 20% (19,20). A recent Danish nation-wide paediatric study reported diminishing colectomy rates [hazard ratio (HR) 0.64] associated with increase in anti-TNF use in children with UC (21).
In this chapter, we review recent data on predictors of poor outcomes in paediatric-onset IBD in the current era of increasing use of biologic therapies. We also discuss emerging data supporting an early intensive approach targeting deeper healing, aiming for remission beyond symptoms with endoscopic healing, radiological healing and biomarker remission emerging as new treatment end points.
Defining a disabling childhood onset IBD
While intensity of symptoms such as diarrhoea, abdominal pain, fatigue is associated with poor quality of life at a given point in time, symptom severity alone at diagnosis is not necessarily associated with poor long-term outcomes in CD (22-24). There is poor correlation between symptoms and endoscopically measured inflammatory disease burden in children with IBD (25,26). It is therefore important to understand biological factors contributing to the risk of complications such as fibro-stenotic, penetrating CD.
There is no universally accepted definition of disabling childhood-onset IBD, but experts define frequent relapse, severe growth delay, development of penetrating and/or stricturing or perianal disease and need for surgery as aggressive PO-CD (27). Similarly, aggressive UC is referred as one that is associated with high relapsing rate (≥2 courses of steroids or hospitalisation after initial diagnosis of UC in the first year), development of colon cancer, presence of extraintestinal manifestation (EIM) and need for colectomy.
Factors associated with complicated CD (Figure 1)
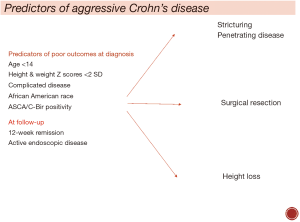
Data from EPIMAD registry suggest that clinical predictors at diagnosis alone are insufficient in predicting disabling CD in children, but age <14, low height Z score <2 standard deviation (SD) and complicated phenotype at diagnosis are poor outcome predictors (28). There are several recent large prospective studies examining factors associated with a complicated course of CD. A prospective multicentre paediatric study recently examined clinical, serological, genetic and microbiome factors associated with complicated CD, defined as change from inflammatory phenotype at diagnosis to a complicated stricturing and fistulising disease (22). At a median follow-up of 3 years, 78/913 (8.5%) developed complicated disease. African American race, anti-Saccharomyces cerevisiae antibodies (ASCA) IgA and C-Bir 1 serology were associated with nearly three times higher risk of penetrating disease (HR 3.0). Stricturing phenotype was associated with ASCA IgA, C-Bir 1 serology and Ileum extra cellular matrix genetic signatures (22). In another recent large study of 1,442 children with CD, clinical factors associated with risk of intestinal resection were examined (29). Authors reported a 25% actuarial risk of intestinal resection at 10 years with complicating phenotype at diagnosis as the only clinical predictor associated with surgical resection (29). A recent European cohort study confirmed that early non-sustained response to treatment is more important than baseline disease severity in CD, with three times higher risk of complications (29%) in those with early relapse vs. 10% in those who sustained remission (23).
Factors associated with poor outcomes in UC (Figure 2)
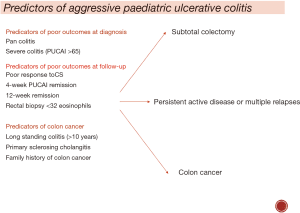
Extensive disease distribution, severity of symptoms and presence of extra-intestinal manifestations at diagnosis are associated with increased risk of colectomy, but the evidence is conflicting. While, some paediatric studies report pan-colitis at diagnosis is a risk factor of colectomy, many studies report no significant association between disease extent and colectomy (30-33).
The best validated index for reporting severity of symptoms is the Pediatric Ulcerative Colitis Activity Index (PUCAI) (30). It is calculated by adding a total score of following six items (abdominal pain, degree of rectal bleeding, stool consistency, number of stools per 24 hours, nocturnal stooling, and activity level). A higher PUCAI at diagnosis is linked with higher risk of colectomy (20). In contrast, Schechter et al did not find this association; they reported a lower CS-free remission (9% vs. 48%, P=0.0001) & higher colectomy rates (6.5% with mild & 24% with moderate activity) in those with 3-month PUCAI >10 vs. PUCAI <10 (34).
These observations suggest that early response to treatment is a very strong predictor of longitudinal outcomes in children with UC and heterogeneity in timings and threshold to introduce effective therapies have a strong influence on the natural history of paediatric UC. A recently concluded large paediatric multicentre inception cohort study using a standardised treatment protocol for therapy escalation overcomes these limitations (35). The authors of this study reported disappointingly low rates of 1 year CS-free remission (defined as PUCAI <10) on mesalazine alone: approaching 50% in those with mild disease and only 30% in those with moderate disease at diagnosis. Dismal outcomes were seen despite additional therapies as almost 50% required additional therapy +/− colectomy within the first year with high biologics and/or immune-modulator use in those with initial moderate vs. mild activity (64% vs. 28%, P<0.001). High initial Mayo score >10, erythrocyte sedimentation rate (ESR) >40 and absence of week 4 PUCAI remission were noted to be the best predictors for colectomy. This study again reminds us that severe presentation and early poor response are important determinants of poor outcomes in UC.
Can early aggressive therapy prevent complications in paediatric IBD?
The understanding that PO-CD is more severe compared to adults has led to several studies examining role of earlier intensive therapies in PO-CD. There is limited paediatric data on long-term efficacy and benefits of early intensive therapy using anti-TNFs (within 3 months of diagnosis) alone or in combination with conventional immune-modulator therapy. Conventional paediatric practice utilizes a step-up approach with anti-TNF agents generally introduced for moderate to severe CD after failure of CS or exclusive enteral nutrition (EEN) and conventional immunomodulators. There are clear benefits of early anti-TNF in children with CD with higher rates of CS-free remission, mucosal healing (MH) and a modest improvement in linear growth. Data on long-term benefits such as intestinal resection or development of complicating disease were lacking up until recently (36-38).
The RISK cohort study is a prospective multicentre paediatric study that examined clinical, serological, genetic and microbiome factors associated with complicated CD, defined as change from inflammatory phenotype at diagnosis to a complicated structuring and fistulising disease (22). Propensity matched adjusted analysis did not confirm a clear benefit of early anti-TNF agents on complicating fibro-stenotic disease but a three-fold reduction in penetrating CD was reported. To conclude that early anti-TNF agents should be routinely used to reduce penetrating disease in CD would be inappropriate as the absolute number of children developing penetrating complications in adjusted analysis were small (15/382, 4%) with 4/15 in the early anti-TNF group developing penetrating disease compared to 11/15 in the group where anti-TNF was introduced at a later stage (22).
Another recent large study including 1,442 children with CD examined clinical factors associated with surgical resection and investigated if early use of anti-TNF reduced the need for surgical resection (29). There were number of interesting observations made in this study. Firstly, in a real-world paediatric study, only a small proportion of patients received early biologics therapy (11% in this cohort), and the majority still received step up anti-TNF therapy. Secondly, there was no clear beneficial effect of early anti-TNFs on progression to surgery or disease behaviour. Furthermore, 5-year surgical resection rates in patients enrolled from 2002 to 2007 and from 2008 to 2014 were identical, despite greater and earlier use of biologics (29).
Can early intensive therapy reduce colectomy rates in UC?
Risk of urgent colectomy in children presenting with acute severe colitis is very high, particularly in those not responding to intravenous (IV) CS. Second-line rescue therapies should be introduced in non-responders or poor responders to CS within 3–5 days of admission as delaying increases the risk of morbidity (33). Anti-TNF agents and calcineurin inhibitors are the main second line rescue therapies in acute severe colitis; their timely use may be associated with a 40% reduction in 1-year colectomy rates from 60% in early 1990’s to 18–22% more recently (39). In children with moderate to severe colitis, anti-TNF use is associated with higher 1- and 2-year CS free remission rates of 38% and 22% and likelihood of remaining colectomy free at 2 years as 41% (39). In this cohort study, only 10% of the total cohort were on biologics by 1 year. Data from recent studies using stringent treatment targets would suggest 40% of children initially presenting with moderate to severe UC would be on biologics within the first year of diagnosis with 10% risk of 1-year colectomy in this group (35). A recently published Danish population-based registry suggests a significant decline in 5-year colectomy rates since introduction of anti-TNF agents in mid-2005 (HR 0.36) (22). Although these studies confirm a trend of increasing use of anti-TNF drugs and a parallel reduction in colectomy rates, the data is insufficient to support the claim that routine early anti-TNF use (within 3 months) in steroid dependent paediatric UC can reduce future risk of colectomy.
Revised treatment targets for CD
“The primary goal of treating patients with IBD is to maximize long-term health-related quality of life through control of symptoms, prevention of structural damage, normalization of function and participation in social and work-related activities.” (40) The concept that a tighter control of chronic diseases can reduce end organ damage is not novel and is widely used in other chronic diseases such as diabetes and hypertension. To define appropriate treatment targets for IBD, we need to not only control symptoms as they impact immediate quality of life but also include objective markers of reducing inflammation as uncontrolled inflammation ultimately leads to cumulative intestinal damage. International evidence-based expert guidelines recommend that the combination of clinical remission and endoscopic remission should be the target in IBD (41). A recently concluded adult study compared a conventional treatment strategy focusing on symptom control versus a tight control arm aiming for symptoms and biomarkers control using C-reactive protein (CRP) and faecal calprotectin (FC) (42). The authors of this study reported a 16% greater improvement in endoscopic remission rates in the tight control group (46% vs. 30% in conventional treatment arm. The trade-off for better endoscopic remission in this study was a greater use of high dose anti-TNFs (46% every week adalimumab vs. 15% every week adalimumab) in the tight control vs. conventional group (42).
It’s time to embrace a treat to target approach but it remains unclear if therapeutic adjustments based on biomarkers are superior to repeated endoscopic examination in achieving MH. On one hand, FC and CRP are excellent non-invasive biomarkers but up to 20% patients with a normal CRP <5 mg/L and FC <250 µg/g of stools at 48 weeks failed to achieve MH [Crohn’s Disease Endoscopic Index of Severity (CDEIS) <4] in the CALM study (43). The other important issue is that escalation of therapies based on biomarkers alone may result in overtreatment of a substantial proportion of patients. Repeat endoscopic evaluation to make treatment adjustments aiming for MH is less well studied but emerging data suggest that this approach may be feasible and is associated with superior outcomes if escalation of therapy is performed in those failing to achieve MH (44,45). Recently published expert paediatric guidelines suggest that repeat endoscopic examination for MH is helpful but it does carry a risk associated with multiple general anaesthetics and health care burden (46). Another feasible and less invasive approach is using an ingestible endoscopic video recording device, such as wireless capsule endoscopy. A recent prospective Italian study utilised pan-enteric capsule endoscopy to guide treatment targets (47). Future trials need to evaluate whether treating to an endoscopic endpoint is better than a biomarker-based approach.
Conclusions
Therapeutic targets in IBD should be individualised after careful evaluation of risk factors with use of early intensive therapy in those considered as high risk, and a timely step-up approach in those with milder forms of disease to minimise medication-related adverse events and maximise long-term quality of life.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author received lecture fees from Abbvie Australia.
References
- Boyapati R, Satsangi J, Gwo-Tzer H, et al. Pathogenesis of Crohn’s Disease. F1000Prime Rep 2015;7:44. [Crossref] [PubMed]
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769-78. [Crossref] [PubMed]
- Duricova D, Burisch J, Jess T, et al. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis 2014;8:1351-61. [Crossref] [PubMed]
- Olén O, Askling J, Sachs M, et al. Childhood onset inflammatory bowel disease and risk of cancer: a Swedish nationwide cohort study 1964-2014. BMJ 2017;358:j3951. [Crossref] [PubMed]
- Vernier-Massouille G, Balde M, Salleron J, et al. Natural History of Pediatric Crohn's Disease: A Population-Based Cohort Study. Gastroenterology 2008;135:1106-13. [Crossref] [PubMed]
- Van Limbergen J, Russell RK, Drummond HE, et al. Definition of Phenotypic Characteristics of Childhood-Onset Inflammatory Bowel Disease. Gastroenterology 2008;135:1114-22. [Crossref] [PubMed]
- Pigneur B, Seksik P, Viola S, et al. Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010;16:953-61. [Crossref] [PubMed]
- Ruemmele FM, Turner D. Differences in the management of pediatric and adult onset ulcerative colitis--lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of pediatric ulcerative colitis. J Crohns Colitis 2014;8:1-4. [Crossref] [PubMed]
- Jakobsen C, Bartek J Jr, Wewer V, et al. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease--a population-based study. Aliment Pharmacol Ther 2011;34:1217-24. [Crossref] [PubMed]
- Jakobsen C, Munkholm P, Paerregaard A, et al. Steroid dependency and pediatric inflammatory bowel disease in the era of immunomodulators--a population-based study. Inflamm Bowel Dis 2011;17:1731-40. [Crossref] [PubMed]
- Hyams J, Markowitz J, Lerer T, et al. The Natural History of Corticosteroid Therapy for Ulcerative Colitis in Children. Clin Gastroenterol Hepatol 2006;4:1118-23. [Crossref] [PubMed]
- Duricova D, Pedersen N, Lenicek M, et al. The clinical implication of drug dependency in children and adults with inflammatory bowel disease: a review. J Crohns Colitis 2011;5:81-90. [Crossref] [PubMed]
- Ramadas AV, Gunesh S, Thomas GA, et al. Natural history of Crohn's disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200-6. [Crossref] [PubMed]
- Nguyen GC, Nugent Z, Shaw S, et al. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology 2011;141:90-7. [Crossref] [PubMed]
- Gupta N, Cohen SA, Bostrom AG, et al. Risk factors for initial surgery in pediatric patients with Crohn’s disease. Gastroenterology 2006;130:1069-77. [Crossref] [PubMed]
- Schaefer ME, Machan JT, Kawatu D, et al. Factors that deter- mine risk for surgery in pediatric patients with Crohn’s disease. Clin Gastroenterol Hepatol 2010;8:789-94. [Crossref] [PubMed]
- Rinawi F, Assa A, Hartman C, et al. Incidence of bowel surgery and associated risk factors in pediatric-onset Crohn’s disease. Inflamm Bowel Dis 2016;22:2917-23. [Crossref] [PubMed]
- Bernstein CN, Ng SC, Lakatos PL, et al. Epidemiology and Natural History Task Force of the International Organization of Inflammatory Bowel Disease (IOIBD). A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis 2013;19:2001-10. [PubMed]
- Fumery M, Duricova D, Gower-Rousseau C, et al. Review article: the natural history of paediatric-onset ulcerative colitis in population-based studies. Aliment Pharmacol Ther 2016;43:346-55. [Crossref] [PubMed]
- Rinawi F, Assa A, Eliakim R, et al. Risk of Colectomy in Patients with Pediatric-onset Ulcerative Colitis. J Pediatr Gastroenterol Nutr 2017;65:410-5. [Crossref] [PubMed]
- Larsen MD, Qvist N, Nielsen J, et al. Use of anti-TNF alpha agents and time to first-time surgery in paediatric patients with ulcerative colitis and Crohn’s disease. J Crohns Colitis 2016;10:650-6. [Crossref] [PubMed]
- Kugathasan S, Denson L, Walters T, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 2017;389:1710-8. [Crossref] [PubMed]
- Ziv-Baran T, Hussey S, Sladek M, et al. Response to treatment is more important than disease severity at diagnosis for prediction of early relapse in new onset pediatric Crohn’s disease. Aliment Pharmacol Ther 2018;48:1242. [Crossref] [PubMed]
- Grover Z, Burgess C, Muir R, et al. Early mucosal healing with Exclusive Enteral Nutrition is associated with improved outcomes in newly diagnosed children with luminal Crohn’s disease. J Crohns Colitis 2016;10:1159-64. [Crossref] [PubMed]
- Zubin G, Peter L. Predicting Endoscopic Crohn's Disease Activity Before and After Induction Therapy in Children: A Comprehensive Assessment of PCDAI, CRP, and Fecal Calprotectin. Inflamm Bowel Dis 2015;21:1386-91. [PubMed]
- Carman N, Tomalty D, Church P, et al. Clinical disease activity and endoscopic severity correlate poorly in children newly diagnosed with Crohn’s disease. Gastrointest Endosc 2019;89:364-372. [Crossref] [PubMed]
- Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179-207. [Crossref] [PubMed]
- Savoye G, Salleron J, Gower-Rousseau C, et al. Clinical predictors at diagnosis of disabling pediatric Crohn's disease. Inflamm Bowel Dis 2012;18:2072-8. [Crossref] [PubMed]
- Kerur B, Machan J, Shapiro J, et al. Biologics Delay Progression of Crohn’s Disease, but Not Early Surgery, in Children. Clin Gastroenterol Hepatol 2018;16:1467-73. [Crossref] [PubMed]
- Turner D, Otley A, Mack D, et al. Development, Validation and Evaluation of a Pediatric Ulcerative Colitis Activity Index: A Prospective Multicentre Study. Gastroenterology 2007;133:423-32. [Crossref] [PubMed]
- Gower-Rousseau C, Dauchet L, Vernier-Massouille G, et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol 2009;104:2080-8. [Crossref] [PubMed]
- Turner D, Walsh CM, Benchimol EI, et al. Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut 2008;57:331-8. [Crossref] [PubMed]
- Turner D, Mack D, Leleiko N, et al. Severe pediatric ulcerative colitis: a prospective multi-center study of outcomes and predictors of response. Gastroenterology 2010;138:2282-91. [Crossref] [PubMed]
- Schechter A, Griffiths C, Gana JC, et al. Early endoscopic, laboratory and clinical predictors of poor disease course in paediatric ulcerative colitis. Gut 2015;64:580-8. [Crossref] [PubMed]
- Hyams JS, David S, Mack DR, et al. Su2017 - Predicting Response to Standardized Pediatric Colitis Therapy: The Protect Study. Gastroenterology 2018;154:S-667. [Crossref]
- Walters TD, Kim MO, Denson LA, et al. Increased effectiveness of early therapy with anti-tumour necrosis factor-α vs an immune-modulator in children with Crohn’s Disease. Gastroenterology 2014;146:383-91. [Crossref] [PubMed]
- Ling J, Buurman D, Ravikumra M, et al. Accelerated Step-Up Infliximab use is associated with sustained primary response in Pediatric Crohn’s disease. Dig Dis Sci 2018;63:1003-10. [Crossref] [PubMed]
- Kang B, Choi S, Kim H, et al. Mucosal Healing in Paediatric Patients with moderate to severe luminal Crohn’s disease under combined Immunosuppression: escalation versus early treatment. J Crohns Colitis 2016;10:1279-86. [Crossref] [PubMed]
- Choshen S, Finnamore H, Auth M, et al. The availability of calcineurin inhibitors and infliximab in acute severe colitis have reduced colectomy rates in 283 children admitted during 1990–2012. J Pediatr Gastroenterol Nutr 2016;62 Suppl 1:13.
- Bossuyt P, Vermeire S. Treat to target in inflammatory bowel disease. Curr Treat Options Gastroenterol 2016;14:61-72. [Crossref] [PubMed]
- Peyrin-Biroulet L, Sandborn W, Sands B, et al. Selecting therapeutic targets in Inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat to target. Am J Gastroenterol 2015;110:1324-38. [Crossref] [PubMed]
- Colombel JF, Panaccione R, Bossuyt P. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2018;390:2779-89. [Crossref] [PubMed]
- Reinisch W, Panaccione R, Bossuyt P, et al. OP015 Biomarker correlation with endoscopic outcomes in patients with Crohn’s disease: data from CALM. J Crohns Colitis 2018;12:S011. [Crossref]
- Bouguen G, Levesque G, Pola S, et al. Endoscopic assessment and treating to target increase likelihood of mucosal healing in patients with Crohns disease. Clin Gastroenterol Hepatol 2014;12:978-85. [Crossref] [PubMed]
- Bouguen G, Levesque BG, Pola S, et al. Feasibility of endoscopic assessment and treating to target to achieve mucosal healing in ulcerative colitis. Inflamm Bowel Dis 2014;20:231-9. [Crossref] [PubMed]
- Oliva S, Thompson M, deRidder L, et al. Endoscopy in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto IBD Group of the ESPGHAN. J Pediatr Gastroenterol Nutr 2018;67:414-30. [Crossref] [PubMed]
- Oliva S, Aloi M, Viola F, et al. A Treat to Target Strategy Using Panenteric Capsule Endoscopy in Pediatric Patients With Crohn's Disease. Clin Gastroenterol Hepatol 2018. [Epub ahead of print]. [Crossref] [PubMed]


