
Pediatric heart failure therapy: why β1-receptor blocker, tissue ACE-I and mineralocorticoid-receptor-blocker?
Definition of heart failure (HF)
HF defines a condition in which the heart is unable to fulfill the body’s need of blood supply. The reason of this inability is either known or idiopathic. Cardiac output is not only a sum of myocardial contractility, heart frequency, heart rhythm, preload and afterload, but is also related to contractile synchrony, interventricular interaction, as well as atrial-ventricular and ventricular-arterial coupling. Therefore, every single component or the sum of all components can lead to an insufficient heart function. Pediatric HF is related to genetic and metabolic abnormalities, structural diseases with volume or pressure overload, hypoxemia, arrhythmias or caused by myocardial disease. Clinical symptoms are dependent on the severity of HF, but also related to age and the cause of HF (1).
New York Heart Association (NYHA) functional classification is not suitable to the young pediatric population (2). Instead, the Ross classification was developed related to the symptoms of infants and young children in term of tachypnea, feeding difficulties, growth problems, sweating and symptoms of exercise intolerance. Additionally, the functional class could be correlated to neurohumoral serum markers as plasma norepinephrine levels (3).
Pathophysiology
Chronic HF is caused by a progressive disease; the consecutive symptoms are related to the endogenous counter-reactions in term of activation of the renin-angiotensin-aldosterone (RAA) as well as sympathetic nervous system. Initially, the water-salt retention and vasoconstriction preserves cardiac output by the Anrep-effect (4) and the Frank-Starling mechanism (5). In acute, these mechanisms restore cardiovascular function with improved symptoms. However, in long-term sustained activation of the neurohumoral axis worsens LV remodeling with loss of functional cardiomyocytes and increased interstitial cardiac fibrosis leading to subsequent systolic and/or diastolic dysfunction lastly to cardiac decompensation with secondary end-organ damage (6).
The pathophysiology of the HF in children differs from that in adults not at least respective to the molecular regulation. A unique characteristic of pediatric dilated cardiomyopathy (DCM) is down regulation of both, β1-AR and of β2-AR, in contrast to DCM in adults where no change of the β2-AR expression is observed (7). Further, molecular regulation, including the potentials of cardiomyocyte proliferation is unique in children; these mechanisms contribute to postnatal myocardial growth. Further, it could be shown, that the percentage of cardiomyocytes in mitosis and cytokinesis is highest in infancy and the number of cardiomyocytes increases until an age of 20 years (8). These results suggest an ability of an age-related myocardial regeneration and a chance for cardiac proliferation in children and still as an adolescent, if stimulated (8). Postnatally, also hemodynamic factors markedly influence myocardial growth; grown-up of borderline left ventricles are related to the amount of intracavity blood flow. In general, pressure load led to both, myocyte hyperplasia/hypertrophy accompanied by myocardial angiogenesis in contrast to only myocyte hypertrophy in a later age (9).
Therapy of HF in children
In history, the most important aim of HF therapy was to improve symptoms, so bedding children in upright position (10) and treating dyspnea and pulmonary congestion by diuretics (11). Continuing to the present, evidence-based clinical trials in children are still missing and recommendations are predicated on clinician’s experience. In contrast, HF treatment in adults is based on guidelines that are periodically reviewed and updated. These guidelines summarize results from studies of large cohorts of homogeneous patient groups (12); consecutively, recommendations are based on multiple randomized trials with a high level of evidence (level A). Difficulty of conducting trials with a sufficient number of pediatric patients result in a lack of evidence-based therapies. Heterogeneous causes of HF in children in a wide range of age do not allow recruiting a sufficient number of patients for randomized trials. Therefore, the few published studies seem to be underpowered. Moreover, the majority of drugs used in adult population have not received a regulatory approval for use in children; 80% of children receive off-label medications for treating HF (13). Neglecting the specific pediatric pharmacokinetics and pharmacodynamics, usually drug levels in children are inadequate or excessive, when adult doses are scaled on the on children’s body weight (14). The largest pediatric HF trial utilizing carvedilol in a multicenter randomized placebo-controlled trial did not show any treatment effect on the end point of clinical HF outcomes. The study enrolled 161 children and adolescents (59% DCM) comparing low- and high-dose carvedilol therapy to placebo on a background of conventional therapy (15). In consequence of this and a few other published results, a recently published Cochrane review concluded, that there are not enough data to recommend or discourage the use of β-blockers in children with HF (16-18). The conclusion, taken only from three studies with a cohort of 20, 22 and 161 patients, respectively, might be one reason, that β-blocker are in general less used by pediatricians. The negative results of the carvedilol study might have a lot of additional causes as the heterogeneity of HF in term of etiology and severity, the used dosages reflecting a strong under-dosing of the utilized β-blocker and may be the inappropriate use of unselective β-blockers (Propranolol or Carvedilol) at least treating pediatric DCM. In context, that infants require in fact fourfold higher doses of for example carvedilol achieving similar drug exposure to adult patients (19), inappropriate diuretic treatment might be an additional limiting factor to establish higher and sufficient dosages of β-blockers, but even ACE-inhibitors.
In fact, the recommended standard pharmacotherapy is unchanged since more than 20 years and is still consisting of diuretics, digoxin and usually (70%) an angiotensin converting enzyme (ACE) inhibitor. Still only 4–18% of pediatric patients with HF undergo therapy with β-blockers (20,21). Among these, more than 70% are treated with carvedilol, despite official FDA reservations considering carvedilol in children (22). This current prescribing trends for chronic HF therapy in children might be even called therapeutic “nihilism” (23).
Child-specific HF therapy
Requirements for specific pediatric HF therapies need to be based on pathophysiology and distinctive features of molecular cardiac profile in children. The aim of pediatric HF-therapy is inhibiting the disease process or creating a chance for cardiac regeneration, if possible. Therefore, clinicians have not further to tailor therapeutic strategies to treat their patient’s symptoms by favoring the triple D-treatment [diuretics, digitalis, diet (fluid restriction)], but should provide therapies affecting adverse biological consequences of sustained neurohormonal activation. Moreover, an important complementary strategy may be based on stimulating endogenous regenerative mechanisms.
Considering the research results of Miyamoto et al. (7), the cardiotoxic effects of endogenously and/or exogenously stimulated β1-ARs have sufficiently to be blocked and contrarily, the cardioprotective properties of β2-ARs be preserved (24); her summary “inhibition of the already down-regulated β2-ARs may override the benefits of β1-AR inhibition in children limiting the efficacy of non-specific blockade in pediatric HF” needs to be taken in account especially treating HF caused by DCM (7). Rather, β1-AR inhibition along with β2-AR stimulation could be useful in acute HF (25,26). Moreover, tachypnea, respiratory problems and coughing are often first symptoms in children with HF and hence unselective β-Blockers may worsen these symptoms, too. The β1- to β2-AR-affinity of bisoprolol is almost 25 times higher to β1-AR in comparison to carvedilol (27). This means that carvedilol needs a much higher dosage for blocking β1-AR comparing to bisoprolol and contemporary blocks the β2-AR. Blocking β2-AR means to lose the advantages of β2-AR preservation in terms of both cardio-protective properties and side-effects, like bronchoconstriction and vasodilative effects. Considering the effectiveness of a drug, obviously it depends on more than just receptor affinity. As mentioned before, age and disease influence the pharmacokinetic profile, absorption behavior, metabolism, tissue distribution and elimination as well as longevity of action at the given receptors. Regarding of this acquired knowledge, the selective long-acting β1-Blocker bisoprolol (B) seems to be the prefers β-Blocker in children with HF, also in consideration of the parent’s compliance, applicating the drug only once per day. Combining bisoprolol with the long-acting tissue angiotensin-converting enzyme (ACE)-inhibitor as lisinopril, further supports this highly important aspect of parent’s compliance: both drugs can be applicated once per day and with a similar dosage of 0.1–0.2 mg/kg per day (28). Considering that angiotensin II, aldosterone and catecholamines mediate trophic stimuli at tissue level and are therefore responsible for the cardiac remodeling, the β1-blocker bisoprolol has to be combined with an ACE-Inhibitor and aldosterone-antagonist. ACE-inhibitors have the properties for reverse remodeling, decreasing systemic vascular resistance and improvement of vascular compliance. The remodeling properties seem to be higher especially with ACE-inhibitors with an effective tissue penetration like Lisinopril (L) (29,30). Compared to tissue-ACE-I, high dosages of serum ACE-I, like Captopril, are needed to inhibit myocardial Angiotensin II formation. High dosages of serum ACE-I induce furthermore bradykinin-dependent side-effects, as bronchoconstriction (symptomatic with cough), especially in young patients. To complete the neurohormonal blockade, an aldosterone antagonist, like spironolactone (S), might be additionally used in a low, non-diuretic dosage, preferentially as an anti-remodeling drug (31).
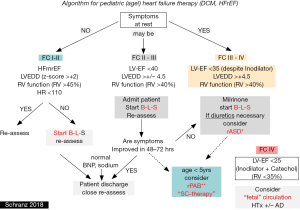
Considering an effective anti-congestive treatment by blocking the sympathetic, RAAS-, and aldosterone system, overtreatment of diuretics should be avoided and diuretics should rather be weaned in long-term therapy, if possible. Inappropriate diuretic treatment forces the neurohumoral axis because of intravascular and in particular intraarterial volume depletion; averts also adequate dosages of Beta-blocker and ACE-I. Additionally, infants with advanced DCM treated by catecholamines can oftentimes not be weaned due to overtreatment of diuretics which leads in some to the inadequate indication for cardiac assist device. Therefore, in most of the admitted young patients with advanced DCM, diuretic treatment needs usually to be weaned, before an adequate B-L-S therapy can be established. Considering further stimulation of the neurohumoral axis by inadequate application of diuretics, the indication for long-term treatment should be strict and not only based on the diagnosis HF (21). In case of a real indicated diuretic treatment, FC III–IV creation of a restrictive interatrial communication should be considered first and may be preferred over a long-term diuretic treatment (Figure 1).
Balanced oxygen consumption and delivery (DO2 = CaO2 × CO) is sine qua none of an optimized HF therapy; achieving an adequate hemoglobin level of 12–14 mg/L belongs to this strategy, but in our opinion also the intermittent application of the cytokine, erythropoietin. Furthermore, erythropoietin seems to have cardioprotective effects beyond of its stimulation of red cell production (34). Known are also stem-cell mobilization and homing properties leading to an increase of endothelial progenitor cells (35). Therefore, we favor erythropoietin as a supportive therapy in particular in young children with DCM.
Considering the regenerative potential of the myocardium in children, which is inversely related to the patients age, all measures favoring regeneration should be considered before cardiac transplantation. In this context it is important to mention, that the left and the right heart do not act in isolation. Observing the nature of congenital heart lesions, the importance of ventricular-ventricular interaction (VVI) and the role of myocardial proliferation properties in young children with the chance for regeneration become obvious (8,36). In fact, VVI plays a pivotal role for heart function in human beings; the right and the left heart are strongly linked together sharing a common ventricular septum, myofibers and pericardium (36,37). Additionally, as mentioned above, pressure overload induces myocyte hyperplasia together with angiogenesis but so far only in young children (9). Based on all of these observations and gained knowledge, pulmonary artery banding (PAB) was introduced as a regenerative therapeutic strategy in infants and young children with advanced DCM provided for cardiac transplantation (32,33,38,39). PAB was aimed to restore LV-geometry and synchrony by leftward shifting of the ventricular septum; VVI-interaction related preload decrease accompanied with a reduced left ventricular end-diastolic volume and pressure. Along with the PAB-induced pressure overload of the right ventricle, myocyte hyperplasia and hypertrophy are stimulated providing the endogenous regenerative ability of the especially young human heart.
Conclusions
Treatment of life-threatening heart insufficiency in children remains a great challenge. Lacking evidence-based clinical trials and missing sufficient pediatric guidelines the therapy remained almost unchanged since decades. One chance for improvement of pediatric HF therapy might be achieved by focusing on the current pathophysiological and pharmacological knowledge. It is hypothesized, that the target therapy should be based on the selective long-acting β1-Blocker Bisoprolol, the long-acting tissue ACE-I Lisinopril and mineralocorticoid-receptor antagonist Spironolactone (named as BLS-therapy).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hsu DT, Pearson GD. Heart Failure in Children Part I: History, Etiology, and Pathophysiology. Circ Heart Fail 2009;2:63-70. [Crossref] [PubMed]
- Association NYH. Diseases of the heart and blood vessels: nomenclature and criteria for diagnosis. Little, Brown, 1964.
- Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol 1992;13:72-5. [Crossref] [PubMed]
- von Anrep G. On the part played by the suprarenals in the normal vascular reactions of the body. J Physiol 1912;45:307-17. [Crossref] [PubMed]
- Katz AM. Ernest Henry Starling, His Predecessors, and the “Law of the Heart.” Circulation 2002;106:2986-92. [Crossref] [PubMed]
- Mann DL, Bristow MR. Mechanisms and Models in Heart Failure The Biomechanical Model and Beyond. Circulation 2005;111:2837-49. [Crossref] [PubMed]
- Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in paediatric idiopathic dilated cardiomyopathy. Eur Heart J 2014;35:33-41. [Crossref] [PubMed]
- Mollova M, Bersell K, Walsh S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci 2013;110:1446-51. [Crossref] [PubMed]
- Di Donato RM, Fujii AM, Jonas RA, et al. Age-dependent ventricular response to pressure overload. Considerations for the arterial switch operation. J Thorac Cardiovasc Surg 1992;104:713-22. [PubMed]
- Kreidberg MB, Chernoff HL, Lopez WL. Treatment of cardiac failure in infancy and childhood. N Engl J Med 1963;268:23-30. [Crossref] [PubMed]
- Engle MA. When the child’s heart fails: Recognition, treatment, prognosis. Prog Cardiovasc Dis 1970;12:601-20. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776-803. [Crossref] [PubMed]
- Pasquali SK, Hall M, Slonim AD, et al. Off-Label Use of Cardiovascular Medications in Children Hospitalized With Congenital and Acquired Heart Disease. Circ Cardiovasc Qual Outcomes 2008;1:74-83. [Crossref] [PubMed]
- Rodriguez W, Selen A, Avant D, et al. Improving Pediatric Dosing Through Pediatric Initiatives: What We Have Learned. Pediatrics 2008;121:530-9. [Crossref] [PubMed]
- Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA 2007;298:1171-9. [Crossref] [PubMed]
- Azeka E, Franchini Ramires JA, Valler C, et al. Delisting of infants and children from the heart transplantation waiting list after carvedilol treatment. J Am Coll Cardiol 2002;40:2034-8. [Crossref] [PubMed]
- Buchhorn R, Hulpke-Wette M, Hilgers R, et al. Propranolol treatment of congestive heart failure in infants with congenital heart disease: The CHF-PRO-INFANT Trial. Int J Cardiol 2001;79:167-73. [Crossref] [PubMed]
- Frobel AK, Hulpke-Wette M, Schmidt KG, et al. Beta-blockers for congestive heart failure in children. Cochrane Database Syst Rev 2009.CD007037. [PubMed]
- Albers S, Meibohm B, Mir TS, et al. Population pharmacokinetics and dose simulation of carvedilol in paediatric patients with congestive heart failure. Br J Clin Pharmacol 2008;65:511-22. [Crossref] [PubMed]
- Harmon WG, Sleeper LA, Cuniberti L, et al. Treating Children With Idiopathic Dilated Cardiomyopathy (from the Pediatric Cardiomyopathy Registry). Am J Cardiol 2009;104:281-6. [Crossref] [PubMed]
- Kantor PF, Lougheed J, Dancea A, et al. Presentation, Diagnosis, and Medical Management of Heart Failure in Children: Canadian Cardiovascular Society Guidelines. Can J Cardiol 2013;29:1535-52. [Crossref] [PubMed]
- Moffett BS, Price JF. National Prescribing Trends for Heart Failure Medications in Children. Congenit Heart Dis 2015;10:78-85. [Crossref] [PubMed]
- Schranz D, Voelkel NF. “Nihilism” of chronic heart failure therapy in children and why effective therapy is withheld. Eur J Pediatr 2016;175:445-55. [Crossref] [PubMed]
- Bernstein D, Fajardo G, Zhao M. The Role of β-adrenergic Receptors in Heart Failure: Differential Regulation of Cardiotoxicity and Cardioprotection. Prog Pediatr Cardiol 2011;31:35-8. [Crossref] [PubMed]
- Xiao RP, Zhu W, Zheng M, et al. Subtype-specific β-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci 2004;25:358-65. [Crossref] [PubMed]
- Navaratnarajah M, Siedlecka U, Ibrahim M, et al. Impact of combined clenbuterol and metoprolol therapy on reverse remodelling during mechanical unloading. PloS One 2014;9:e92909. [Crossref] [PubMed]
- Ladage D, Schwinger RHG, Brixius K. Cardio-selective beta-blocker: pharmacological evidence and their influence on exercise capacity. Cardiovasc Ther 2013;31:76-83. [Crossref] [PubMed]
- Recla S, Steinbrenner B, Schranz D. Medical therapy in dilated cardiomyopathy and pulmonary arterial banding in children. J Heart Lung Transplant 2013;32:1045-6. [Crossref] [PubMed]
- Chrysant SG. Vascular remodeling: The role of angiotensin-converting enzyme inhibitors. Am Heart J 1998;135:S21-30. [Crossref] [PubMed]
- Saha SA, Molnar J, Arora RR. Tissue ACE inhibitors for secondary prevention of cardiovascular disease in patients with preserved left ventricular function: a pooled meta-analysis of randomized placebo-controlled trials. J Cardiovasc Pharmacol Ther 2007;12:192-204. [Crossref] [PubMed]
- Masutani S, Saiki H, Kurishima C, et al. Heart Failure With Preserved Ejection Fraction in Children. Circ J 2013;77. [Crossref] [PubMed]
- Bauer A, Esmaeili A, deRosa R, et al. Restrictive atrial communication in right and left heart failure. Transl Pediatr 2019;8:133-9.
- Michel-Behnke I, Pavo I, Recla S, et al. Regenerative therapies in young hearts with structural or congenital heart disease. Transl Pediatr 2019;8:140-50.
- Burger D, Xenocostas A, Feng QP. Molecular basis of cardioprotection by erythropoietin. Curr Mol Pharmacol 2009;2:56-69. [Crossref] [PubMed]
- Heeschen C, Aicher A, Lehmann R, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 2003;102:1340-6. [Crossref] [PubMed]
- Friedberg MK, Redington AN. Right Versus Left Ventricular Failure Differences, Similarities, and Interactions. Circulation 2014;129:1033-44. [Crossref] [PubMed]
- Smerup M, Nielsen E, Agger P, et al. The Three-Dimensional Arrangement of the Myocytes Aggregated Together Within the Mammalian Ventricular Myocardium. Anat Rec (Hoboken) 2009;292:1-11. [Crossref] [PubMed]
- Schranz D, Rupp S, Müller M, et al. Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: A novel therapeutic strategy before heart transplantation. J Heart Lung Transplant 2013;32:475-81. [Crossref] [PubMed]
- Schranz D, Akintuerk H, Bailey L. Pulmonary Artery Banding for Functional Regeneration of End-Stage Dilated Cardiomyopathy in Young Children: World Network Report. Circulation 2018;137:1410-2. [Crossref] [PubMed]


