Pulmonary artery banding in dilative cardiomyopathy of young children: review and protocol based on the current knowledge
Introduction
Dilated cardiomyopathy (DCM) is still a leading cause of cardiac death in children (1,2). DCM of the left ventricle (LV-DCM) is characterized by LV-ejection fraction (LV-EF) of less than 40% and LV-end-diastolic dimension (LVEDD) with z-values above +2; LVEDD with a z-value of above +5 has a low incidence of spontaneous recovering may be maximal of 10–15% of the patients (2). Idiopathic and myocarditis related LV-DCM is the domain in infants and young children, while in adult’s systolic cardiac dysfunction and dilatation is preferentially based on an ischemic myocardial disease (3). Considering the “traditional” pediatric CHF-therapy as symptom- oriented and non-curative (4,5), report from the working group of the National Institutes of Health reminds major new initiatives for the development of new therapies in children with heart failure (6). Patients with “end-stage” heart failure and maximal guideline-related medical therapy are listed for heart transplantation (HTx), in some also bridged by an assist device. HTx is considered as the only viable life-saving option despite its palliative character with reduced long-term survival (7,8); further, limited by donor availability, its costs and life-long immunosuppressive treatment with its negative consequences in long-term. From global health perspective, HTx is available to only a very small minority of affected children; therefore, there is a pressing need for alternative therapies. Regarding the enormous potentials for cardiac regenerative, reciprocal to the patient’s age, alternatives are obvious and should took into account [Michel-Behnke et al. (9)]. In this context, it has to be considered that left and right ventricle do not act in isolation; they are inextricably linked through a common septum, shared myofibers and pericardial space (10); LV contraction contributes more than 50% of RV work (11). Despite of novel experimental and clinical observations (12-15), the potential’s of ventricular-ventricular (V-V) interactions in either LV or RV failure has not been harnessed for therapeutic benefit. Here, we hypothesize that V-V interactions have enormous therapeutic potential in patients with LV-DCM. Therefore, we introduced reversible pulmonary artery banding (rPAB) to the therapeutic arsenal in left heart failure with reduced ejection fraction (HFrEF), but preserved RV-function; it seems to constitute a paradigm shift, particularly for infants and children with end-stage DCM (16-18). Reversible PAB is a promising therapeutic option with the potential to be a clinical ‘game changer’ (19).
PAB in LV-DCM, why?
The aim of surgical performed PAB is to support functional recovery of LV-DCM.
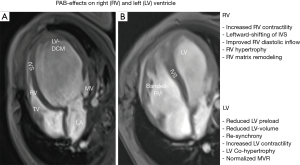
It is hypothesized, that rPAB shifts the interventricular septum back to the left by forcing a pathological, round, “apple-like” left ventricular shape to its normal, ellipsoid “pear-like” morphology (20,21); aiming that the function follows the morphology. Thereby, PAB induced RV stress improves LV filling dynamics as well as end-diastolic volume and pressure via Anrep and Frank-Starling effects (Figure 1A,B). Together with restored ventricular electromechanical synchrony, increased LV ejection fraction and less left atrial congestion, PAB becomes responsible for clinical improvement at rest and during exercise (21). Initially, the idea was born by observing the favourable results of PAB in patients with congenital corrected transposition of the great arteries (ccTGA), in which long-term outcome is directly related to a balanced VVI and the competence of atrio-ventricular valve (22,23). Pathological enlargement of a sub-aortic positioned systemic RV is significantly longer be prevented by a pulmonary or left ventricular out-flow tract obstruction, but even by pulmonary artery hypertension. Surgical PAB is used in case of an unloaded sub-pulmonary positioned LV and thereby missing counter bearing effect avoiding RV dilatation (23). PAB is used for retraining a sub-pulmonary LV, but even as a preventive strategy for neonates born with ccTGA without congenital pulmonary or sub-pulmonary obstruction (24).
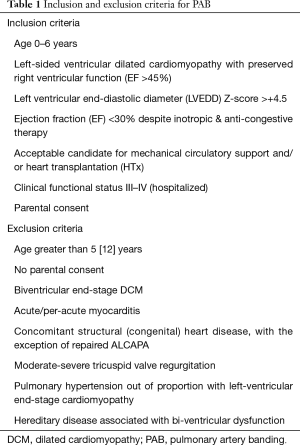
Entry- and exclusion-criteria were developed for young children with LV-DCM and preserved right ventricular function (Table 1); aiming functional recovery, instead HTX or death (17). The efficacy of a placed PAB seems to be reciprocal to the patient’s age because of the endogenous potential’s of myocardiocyte recovery and repopulation (25-27). Animal models with distinction of age have shown that chronic PAB increases myocytal size during RV-hypertrophy accompanied by cell growth directed gene expression pattern together with a parallel augmented capillary network (28,29). Additionally, risk-benefit of surgical PAB seems even be reciprocal to the age particularly in severely diseased patients with LV-DCM. Additionally, young infants have the chance to grow-in the PAB; thereby the RV can smoothly adapt to a systemic pressure level and the LV becomes a high chance for functional recovery (18).
Full table
PAB in LV-DCM, how?
Before a reversible PAB is considered treating a LV-DCM, the diagnosis DCM must be secured. The difference between DCM and a dilated LV caused by pressure (aortic coarctation, critical aortic stenosis) or volume overload (MV regurgitation) can oftentimes be made by the LV contraction behavior in term of a-synchronic versus synchronic contraction profile. Completed diagnostic work-up is mandatory; especially in a young infancy! Clinical and imaging examination have to exclude causative structural heart defects as an aortic coarctation or critical aortic stenosis; MRT imaging can additionally figure out RV function and form (RV-EF) in context of the diseased LV (LV-non compaction criteria, myocarditis; ischemic areas late enhancement? etc.). The determinants of cardiac output needs to be in account; beyond heart rate, contractility preload and afterload, parameters of ventriculo-ventricular interaction, interatrial and atrial-ventricular as well as ventriculo-arterial coupling have to be analyzed.
Heart catheterization need to be additionally performed for excluding coronary anomalies, obstructions, fistulae etc.). Myocardial biopsies or therapeutical issues as creating a restrictive ASD should further be considered as ever possible.
Placing a surgical band around the pulmonary artery trunk is technically simple, the band has to be performed that neither the pulmonary valve nor the bifurcation of pulmonary artery is bothered. Therefore, the band should be additionally fixed at the adventitia of the PA avoiding distal migration. PAB has a low risk in patients with ventricular septum defect to reduce a left-right shunt. Restriction of pulmonary artery blood flow balancing systemic-pulmonary circulations is mostly associated with normal or hyper-dynamic ventricular function. In case of LV-DCM, the PAB approach however needs to be carefully prepared, performed and followed initially by a continuous hemodynamic control.
The pediatric cardiac surgeon, anesthesiologist, intensivist and cardiologist have to have the common sense, that the child should become the chance for cardiac recovering instead HTx. It needs this one focus, if one member of the team is disagreeing, the open-chest method of PAB should not be performed in young children with “end-stage” LV-DCM. One physician, best the pediatric cardiologist familiar with the pathophysiology and peri-operative as well as long-term heart failure therapy should take close care for the patient; coordinating the procedure, which also includes timing of the surgical approach and involving closely the parents in any step of this novel treatment for cardiac recovery.
The summarized methods according our protocol
According the entry & exclusion criteria (Table 1), PAB is currently offered to young children {<6 [12] years} with LV-DCM and preserved RV-EF admitted for HTx or HTx evaluation; the parents have given written informed consent; the history and the treatment at admission is documented, but immediately adapted to the protocol specifications (Tables 2,3), which also respects the receptor-specific differences between DCM in children and adults (30,31). Therefore, all infants and children undergoing a rPAB are being treated with a β1-specific beta-blocker (B, Bisoprolol), a tissue ACE-inhibitor (L, Lisinopril) and a mineralocorticoid-blocker (S, Spironolactone), while we avoid diuretics to the extent it is possible (32,33). Avoiding intra-vascular volume deficit with a risk for hypotension during the establishment of B-L-S or even continuous milrinone infusion, all loop-diuretics have to be stopped as a minimum of 12–24 before this specific treatment is started. Aiming, that surgical PAB needs to be paired with an improved anti-congestive therapy favoring cardiac functional recovery (34). Inhibition of endogenous neuro-humoral activation is only one significant part of cardiac treatment, but protecting cardiomyocytes and cardiac interstitium is a further strategy reducing mechanical stress related fibrosis of the RV by PAB (29,35). Clearly, long-term risks and benefits need to be analyzed in a multicenter study, but all current retrospective data supports the here-recommended “modus operandi”.

Full table

Full table
Assuming the diagnosis “LV-DCM with preserved RV-EF” made by history—age for example 6 months—clinical examination, ECHO, cardiac MRI-imaging as shown in Figure 1A; then assessing normal LA-size, there is obviously no indication for diuretic treatment, as seen in the majority of young patients admitted even for HTx evaluation; in case of an increased heart rate of >140/min with or without concomitant therapy by catecholamines, as it is also usually performed, diuretics need to be stopped, feeding allowed ad libitum, before B. and L. is started with an orally dosage of 0.1 mg/kg each and S. of 0.5–1 mg/kg; all drugs need only applicated once per day. Aiming a heart rate of less than 110/min at rest (sleep) even in young infancy together with a normal breath rate and sufficient systolic and diastolic blood pressure. Sometimes, catecholamines, in particular continuous infusion of epinephrine, can only be stopped and might be changed to milrinone, if a volume challenge is performed by a short infusion (10–20 mL/kg) of Ringer® solution and/or by increasing Hb to 12–14 g% by transfusion of red cell package; both, by careful monitoring the blood pressure, which have to remain stable or increase but not decrease. Cardiac catheterization is followed with coronarography (ALCAPA-exclusion) and myocardial biopsies; myocardial biopsies will be obtained for routine histopathological analysis and viral persistence. Creation of a restrictive atrial septum defect is indicated, when diuretics are really needed treating pulmonary congestion; imaging as well as LA-pressure or PCWP-measuring evidenced the indication creating a restrictive atrial septum defect (36,37).
Counseling worldwide physicians with the question “is our patient a candidate for PAB?” our answer is usually as following: please sent history, age, current treatment, echocardiographic data as movies of the four-chamber, long- and short-axis view; LVEDD z-score as well as LV- and RV-EF, obtained best by MRI, if available.
PAB-procedure briefing step-by-step (based on a usually referred infant-patient)
Prior to surgical PAB appropriate interval during follow-up surveillance is allowed to prepare “listing” for HTx and establishing perioperative medical therapy. Already established, long-acting bisoprolol is applicated about 6 to 12 hours before surgery for reversible PAB is provided; continuous intravenous infusion of milrinone is usually established together with B-L-S days before in a dosage, adapted to 1 µg/kg × min. Twelve hours before surgical PAB, levosimendan has additionally be infused in a dosage of 0.1 µg/kg × min without loading dose; the infusion should be continued for further 12 hours during anesthesia for PAB surgery and on PICU. Reversible PAB is also being applied even in lieu of mechanical circulatory support (left-heart assist device) or for weaning of already existing extracorporeal mechanical oxygen (ECMO).
Open chest surgery is performed under continuous monitoring of heart rate (HR), systemic atrial blood pressure (SAP), right atrial pressure (RAP), pulse oximetric oxygen saturation and cerebral near infrared spectroscopy (cNIRS) calibrated to oxygen saturation obtained from the upper caval vein. Anesthesia is mostly performed by midazolam, fentanyl, vecuronium in a continuous infusion and small single dosages, if needed; additionally, continuous infusion of clonidine (1–2 mg/kg/hour) is established or continued; infusion of epinephrine (0.01–0.05 µg/kg/min) and norepinephrine (0.01–0.2 µg/kg/min) are prepared to start several minutes prior to PAB-application, if not already applicated for treatment of cardiac failure before.
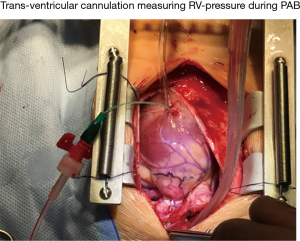
A sternotomy and partial pericardia incision is used placing the rPAB. It has to be aware, that the V-V-I is immediately changed, when the pericardium is opened (blood pressure decrease, heart rate increase!). Therefore, the surgical approach should be well prepared (volume, Hb!) and shortened as possible; the pericardium needs to be carefully closed at the end of the procedure, again by close hemodynamic monitoring. A polyethylene 21G (arterial) cannula is placed within the right ventricle by transmural puncture, and fixed by a purse-string suture (Figure 2). The band has to be placed carefully around the pulmonary arterial trunk without compromising the systemic and in particular coronary blood flow; before the PAB is tightens, hemodynamic and oxygen transport parameters have to be stable:
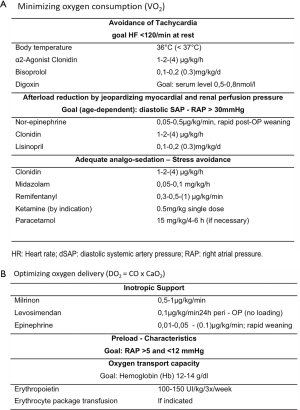
- Heart rate (HR, influenced by long-acting bisoprolol, and clonidine infusion) should be less than 145/min, preferred <125/min despite catecholamine support;
- Right atrial pressure (RAP) need to be above 5 mmHg (sufficient intravascular volume and hemoglobin >12 g%), but less than 12 mmHg;
- Systolic arterial pressure (SAP)—age- and anesthesia-dependent—should be above 70–[80] mmHg, if the RVP is less than 35 mmHg;
- Arterial-venous oxygen saturation difference (Sa-vDO2) should be <40%, preferred <30%;
- TAPSE (tricuspid annular plane systolic excursion), degree of the mitral valve regurgitation (MR) as well as the interventricular septal position (IVS) are additionally monitored by TEE, all TEE- parameters should analyzed before, during, after PAB.
- LAP-RAP gradient should also be determinated by TEE, in case of persistent foramen ovale or percutaneous created restrictive ASD.
Tightening of the already under the PA placed band (PTFE or Dacron) should be performed smoothly under continuous hemodynamic monitoring and TEE control until the IVS becomes slightly shifted leftwards. Usually, the PA-circumference can be reduced to about 50–60% of the diameter of the pulmonary valve annulus without jeopardizing SAP or an increasing RAP above 10 mmHg; depending on the PAB-induced decrease of TAPSE, a systolic RVP/SAP ratio of 0.6 might be achieved; the pressure gradient across the PAB immediately after band placement is usually “only” 20–25 [35] mmHg; the true pressure gradient can be measured, when the RV-function is re-adapted (TAPSE back to basic value), mostly seen just before the patient is discharged home. Considering the reversibility of the PA-band, two sutures with prolene 6.0 (5.0) are used allowing the chance for partial or complete de-banding by transcatheter balloon dilatation; a further prolene suture is placed 3–5 mm above the first double suture-line giving the chance not only for patient’s grown-in the PAB, but even to establish a live-long residual band-effect, which might be necessary and we favor in LV-DCM associated with a non-compaction morphology.
During open chest PAB approach, myocardial function is augmented with catecholamines and inodilators (see above); once the postoperative hemodynamics are instable in particular when systemic hypotension is induced by patient’s agitation, the patient needs be muscle relaxed until stability is established. However, usually the patient is extubated early after surgery and cardiac protecting drugs B-L-S are early re-established, even still during continuous catecholamine support (Tables 2,3; Figure 3). The highly specific β-1-adrenoreceptor blocker bisoprolol or the intravenous variant metoprolol or esmolol should already protect against the endogenous and exogenous β1-adrenergic stimulation with deleterious cardiac effects (myocyte apoptosis, necrosis); β2-receptors with its quite different receptor-related pathway should however be preserved considering its cardiac protective, hypertrophy supporting and stem cell mobilizing effects (31); possible (β2-R) side effect, if additionally stimulated by continuous β2-agonists in particular its chronotrope action can sufficiently be blocked by sufficient β1-receptor blockers combined with the anti-tachycardia effects of clonidine; in some patients digoxin or even ivabradin (0.1 mg/kg) are additionally used. As mentioned above, catecholamines have to wean off gradually over a short period of days (TAPSE!!), when milrinone is still continuously infused; milrinone is weaned over 1–2 (–4) weeks most by deceasing the dosage of 0.1 µg/kg × min per day by monitoring the clinical condition (Ross-status, and BNP-values). The infants are separated from mechanical ventilation within the first post-rPAB day(s) or seldom week(s), and enteric feeding is resumed immediately given all recommended oral drugs back in a sufficient dosage (Table 2). The infant is discharged on oral medications (B-L-S) by avoiding furosemide, but in some by low-dosage of hydrochlorothiazide (0.5–1 mg/kg) in 1 or 2 oral applications per day; supplemental drugs are applicated long-term (Table 3); erythropoietin, which is administered 3×/weeks in a dosage of 100–150 IE/kg iv/im as long as the patient is not discharged home. Every child is kept under frequent clinical surveillance. The infants’ short- and long-term surveillance will include parent’s education to monitor/observe the breath rate at sleep and by taught parents applicate safely the oral drugs, in particular the three most important one B-L-S once per day; the parental compliance effect is usually excellent. Intermittent clinical examinations in close contact to the Pediatric Heart C enter have not only monitored height, weight, electrocardiogram, echocardiogram, cardiac MRI, plasma BNP levels, but even the parent’s reported history; the indication for an invasive examination is ordered by the responsible Heart Center, in particular heart catheterization for re-myocardial biopsies or PAB de-banding by PAB-ballooning.
PAB in LV-DCM; how long?
The first rPAB approach to treat LV-DCM was performed meanwhile 12 years ago, when the patient was listed as a newborn for HTX over 2 months without a realistic chance receiving a donor heart (16). Meanwhile, he is de-banded since years by transcatheter technique and still living with a NYHA functional class I without limited cardiac function despite minimal morphological changes in term of signs of bi-ventricular hypertrophy. Considering the last update, published as worldwide retrospective data analysis (18), it can be assumed, that more than 100 infants and young children have currently undergone rPAB with end-stage DCM for bridging to HTx or functional recovery. To best of our knowledge, PAB-procedure related mortality is not described until now. PAB-responder, remarkable clinical and morphological improvements of the LV cardiac function with a reverse remodeling in term of normalization of LV-size, LV-ejection fraction, and mitral valve regurgitation is usually observed between 3 to 9 months after PAB-placement (Figure 1B). The majority of patients could be de-listed from transplant list. Following further improvement in their clinical and hemodynamic status, complete or partial de-banding of the pulmonary artery can be performed by transcatheter technique utilizing a gradual balloon dilatation by utilizing high-pressure balloons. Based on our first, negative experience in DCM-patients associated with a non-compaction LV morphology (17,18), in who complete transcatheter de-banding led to cardiac decompensation despite a fantastic initial response on PAB with normalizing LV form and function, we decided against complete RV-unloading. Since these initial experiences, we led a residual RV hypertension of 50–60% of the systemic level in all patients with non-compaction morphology, even when the LV recovered fully during the follow-up. Complete or even partial transcatheter de-banding can be easily performed, if the above described surgical PAB-technique is used. The indication for PAB-debanding is made, when clinical signs of exercise-intolerance becomes obvious despite a well functional LV. Usually, RV-distress with ventricular dilatation and high pressure gradient across PAB is usually observed together with a moderate tricuspid valve regurgitation and secondary increase of plasma B-type natriuretic peptide (BNP). Band enlargement may be accomplished in stages, ultimately opting for a mild residual right ventricle-main pulmonary artery pressure gradient of 15–30 mmHg. As mentioned above, he technique of PAB-ballooning depends on the annulus of the pulmonary valve; we don’t use balloons with a diameter above the PA-valve annulus; we starting with a high-pressure balloon with diameter of about 50% larger than the smallest measured PAB-diameter (for example 4–5 mm PAB vs. 8 mm balloon diameter); followed by a bigger balloon, when re-angiography and pressure gradient measurement are performed again and in consideration of the final aim to achieve a partial of complete PA-debanding.
PAB in LV-DCM: preliminary conclusion
Usually, left and right heart failure is considered separately, but from the pathophysiological point of view, both, right and left heart function is inextricably linked through a common septum, shared myofibers and pericardium. Application of a reversible PAB utilizes great potentials of VVI to treat LV failure with reduced ejection fraction (HFrEF). First ‘proof-of-principle’ results imply that rPAB in LV failure provides a novel alternative ‘bridge-to-transplant’ or destination therapy in infants and children with advanced LV-DCM. Technically, rPAB is simple, safe, effective and affordable making it a realistic option for children worldwide, especially where transplant is not an option. In young infants with the potentials of regeneration; with the option to grown-in the PAB, the surgical PAB can be performed with very low risk, but highly effective; there is no need for a tight PAB, as it was shown even in ccTGA patients in whom a banding is considered in young infancy as prophylactic tool. Independent of the patient’s age, a provided PAB procedure needs to be performed as standardized measure independent if the perioperative strategy is considered as low- or high-risk approach. In any case, pathophysiological aspects and the current pharmacological knowledge of a patient designed pediatric heart failure therapy have to be taken in account. Currently, we have demonstrated therapeutic benefit only in infants and young children whose myocardium may have high potential for regeneration; however, the pediatric DCM population in total has not been clearly defined who may benefit from this novel V-V-interactive procedure. The need for a prospective, randomized study is obvious to evidence rPAB in LV-DCM; clinical outcomes with biochemical profiling are warranted as well as the impact of PAB to diminish the need for mechanical circulatory support and cardiac transplantation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Daubeney PE, Nugent AW, Chondros P, et al. Clinical features and outcomes of childhood dilated cardiomyopathy: results from a national population-based study. Circulation 2006;114:2671-8. [Crossref] [PubMed]
- Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006;296:1867-76. [Crossref] [PubMed]
- Patel MD, Mohan J, Schneider C, et al. Pediatric and adult dilated cardiomyopathy represent distinct pathological entities. JCI Insight 2017. [Crossref] [PubMed]
- Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 2007;298:1171-9. [Crossref] [PubMed]
- Roche SL, Redington AN. Right ventricle: wrong targets? Another blow for pharmacotherapy in congenital heart diseases. Circulation 2013;127:314-6. [Crossref] [PubMed]
- Burns KM, Byrne BJ, Gelb BD, Kühn B, et al. New mechanistic and therapeutic targets for pediatric heart failure: report from a national heart, lung, and blood institute working group. Circulation 2014;130:79-86. [Crossref] [PubMed]
- Alexander PM, Daubeney PE, Nugent AW, et al. Long-term outcomes of dilated cardiomyopathy diagnosed during childhood: results from a national population-based study of childhood cardiomyopathy. Circulation 2013;128:2039-46. [Crossref] [PubMed]
- Canter CE, Shaddy RE, Bernstein D, et al. Indications for heart transplantation in pediatric heart disease: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young; the Councils on Clinical Cardiology, Cardiovascular Nursing, and Cardiovascular Surgery and Anesthesia; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007;115:658-76. [Crossref] [PubMed]
- Michel-Behnke I, Pavo I, Recla S, et al. Regenerative therapies in young hearts with structural or congenital heart disease. Transl Pediatr 2019. [Epub ahead of print].
- Sanchez-Quintana D, Anderson RH, Ho SY. Ventricular myoarchitecture in tetralogy of Fallot. Heart 1996;76:280-6. [Crossref] [PubMed]
- Damiano RJ Jr, La Follette P Jr, Cox JL, et al. Significant left ventricular contribution to right ventricular systolic function. Am J Physiol 1991;261:H1514-24. [PubMed]
- Yamashita H, Onodera S, Imamoto T, et al. Functional and geometrical interference and interdependency between the right and left ventricle in cor pulmonale: an experimental study on simultaneous measurement of biventricular geometry of acute right ventricular pressure overload. Jpn Circ J 1989;53:1237-44. [Crossref] [PubMed]
- Belenkie I, Horne SG, Dani R, et al. Effects of aortic constriction during experimental acute right ventricular pressure loading. Further insights into diastolic and systolic ventricular interaction. Circulation 1995;92:546-54. [Crossref] [PubMed]
- Apitz C, Honjo O, Friedberg MK, et al. Beneficial effects of vasopressors on right ventricular function in experimental acute right ventricular failure in a rabbit model. Thorac Cardiovasc Surg 2012;60:17-23. [Crossref] [PubMed]
- Apitz C, Honjo O, Humpl T, et al. Biventricular structural and functional responses to aortic constriction in a rabbit model of chronic right ventricular pressure overload. J Thorac Cardiovasc Surg 2012;144:1494-501. [Crossref] [PubMed]
- Schranz D, Veldman A, Bartram U, et al. Pulmonary artery banding for idiopathic dilative cardiomyopathy: a novel therapeutic strategy using an old surgical procedure. J Thorac Cardiovasc Surg 2007;134:796-7. [Crossref] [PubMed]
- Schranz D, Rupp S, Muller M, et al. Pulmonary artery banding in infants and young children with left ventricular dilated cardiomyopathy: A novel therapeutic strategy before heart transplantation. J Heart Lung Transplant 2013;32:475-81. [Crossref] [PubMed]
- Schranz D, Akintuerk H, Bailey L. Pulmonary artery banding for functional regeneration of end-stage dilated cardiomyopathy in young children: World network report. Circulation 2018;137:1410-2. [Crossref] [PubMed]
- Bailey LL. Back to the future! Bold new indication for pulmonary artery banding. J Heart Lung Transplant 2013;32:482-3. [Crossref] [PubMed]
- Schranz D, Akintuerk H, Voelkel NF. "End-stage" heart failure therapy: from pulmonary artery banding and interatrial communication to parallel circulation. Heart 2017;103:262-7. [Crossref] [PubMed]
- Latus H, Hachmann P, Gummel K, et al. Biventricular response to pulmonary artery banding in children with dilated cardiomyopathy. J Heart Lung Transplant 2016;35:934-8. [Crossref] [PubMed]
- Graham TP Jr, Bernard YD, Mellen BG, et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol 2000;36:255-61. [Crossref] [PubMed]
- Winlaw DS, McGuirk SP, Balmer C, et al. Intention-to-treat analysis of pulmonary artery banding in conditions with a morphological right ventricle in the systemic circulation with a 540 view to anatomic biventricular repair. Circulation 2005;111:405-11. [Crossref] [PubMed]
- Metton O, Gaudin R, Ou P, et al. Early prophylactic pulmonary artery banding in isolated congenitally corrected transposition of the great arteries. Eur J Cardiothorac Surg 2010;38:728-34. [Crossref] [PubMed]
- Amir G, Ma X, Reddy VM, et al. Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg 2008;86:1311-9. [Crossref] [PubMed]
- Mishra R, Vijayan K, Colletti EJ, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation 2011;123:364-73. [Crossref] [PubMed]
- Mollova M, Bersella K, Walsha S, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci U S A 2013;110:1446-51. [Crossref] [PubMed]
- Takeda N, Manabe I, Uchino Y, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest 2010;120:254-65. [Crossref] [PubMed]
- Friedberg MK, Cho MY, Li J, et al. Adverse Biventricular Remodeling in Isolated Right Ventricular Hypertension is Mediated by Increased TGFβ1 Signaling and is Abrogated by Angiotensin Receptor Blockade. Am J Respir Cell Mol Biol 2013;49:1019-28. [Crossref] [PubMed]
- Miyamoto SD, Stauffer BL, Nakano S, et al. Beta-adrenergic adaptation in pediatric idiopathic dilated cardiomyopathy. Eur Heart J 2014;35:33-41. [Crossref] [PubMed]
- Recla S, Steinbrenner B, Schranz D. Medical therapy in dilated cardiomyopathy and pulmonary arterial banding in children. J Heart Lung Transplant 2013;32:1045-6. [Crossref] [PubMed]
- Schranz D, Voelkel N. Nihilism of chronic heart failure therapy in children and why effective therapy is withheld. Eur J Pediatr 2016;175:445-55. [Crossref] [PubMed]
- Schranz D. Diuretic Treatment in Heart Failure. N Engl J Med 201815;378:683
- Recla S, Schmidt D, Logeswaran T, et al. Pediatric heart failure therapy: why β1-receptor blocker, tissue ACE-I and mineralocorticoid-receptor blocker? Transl Pediatr 2019. [Epub ahead of print].
- Bogaard HJ, Natarajan R, Henderson SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009;120:1951-60. [Crossref] [PubMed]
- Bauer A, Khalil M, Schmidt D, et al. Transcatheter left atrial decompression in patients with dilated cardiomyopathy: bridging to cardiac transplantation or recovery. Cardiol Young 2019;29:355-62. [Crossref] [PubMed]
- Bauer A, Esmaeili A, deRosa R, et al. Restrictive atrial communication in right and left heart failure. Transl Pediatr 2019. [Epub ahead of print].