
Ideal intensive care unit course following comprehensive stage II in hypoplastic left heart syndrome
Introduction
Since William Norwood introduced 1983 (1) a reconstructive palliation for Hypoplastic Left Heart Syndrome (HLHS), the “Norwood” procedure developed to the standard procedure worldwide. A second stage is performed at an age of 4–6 months, connecting the upper caval vein as a bi-directional Glenn-anastomosis; after all, the Fontan procedure completes the staged procedure in an age between 2 and 4 years. From 1985 neonatal cardiac allotransplantation (HTX), first accomplished by Leonard Bailey, marked an alternative palliation (2). At the Pediatric Heart Center of the University Hospital Giessen HTX was introduced 1988 as the primary palliation, followed by the Norwood procedure in 1993 (3). Based on the first trials by John Gibbs in Leeds (4), we were able to perform the first successful Hybrid staged approach in 1998 (5,6). Initially, the Hybrid approach consisting of postnatal bi-lateral pulmonary banding (bPAB), percutaneous ductal stenting and in case of need manipulation of the atrial septum, was performed as a rescue measure (5). However, when the surgical comprehensive stage II became to a routine (7), it replaced the Norwood procedure despite its even advanced technique (8). Comparing Hybrid versus Norwood approach, the fundamental difference between both strategies consists of the risks and complications resulting from cardiopulmonary bypass (CPB) and the shunt dependent pulmonary blood flow. In this context the unsolved problems of neonatal circulatory arrest in terms of neurological side effects, particularly for neonates with a univentricular physiology and parallel connected pulmonary and systemic circulation, must be considered (9,10). The Hybrid approach delays an extensive open-heart surgery from the vulnerable neonatal period into later life between 4 and 6 months of age (11,12)—a fact that must be seen beyond the overall mortality and morbidity but in consideration of the unfavorably long-term success. The components of the Hybrid stage I are even the most effective treatment for resuscitating a neonate with HLHS admitted in cardiogenic shock (13). Comprehensive stage II consists of bilateral pulmonary de-banding, removal of the stented duct, Norwood-like reconstruction of the ascending aorta and aortic arch as well as establishing a bidirectional cavopulmonary connection with left pulmonary artery (LPA) patch reconstruction or stenting (11). Published mortality rates of stage II operation range from 4–9% (14,15). Optimizing the long-term outcome of the Hybrid strategy in patients with HLHS might be in particular depending on further improvements of the comprehensive stage II including the anesthesiologic management and immediate post-operative intensive care (14). In this report we present an almost ideal intensive care course following comprehensive stage II surgery; aiming to offer knowledge exchange and support for other centers worldwide dealing with HLHS by a Hybrid approach.
Case presentation
A girl with HLHS (mitral stenosis, aortic atresia) and body weight of 3.1 kg underwent off-pump bPAB banding at day 5 of life; the bPAB was performed by cutting a 3.5-mm polytetrafluoroethylene (PTFE) tube (11); interventional duct stenting was performed as a percutaneous transcatheter approach 2 days after bPAB as a an elective procedure utilizing a self-expandable Sinus-Superflex-DS-Stent (7 mm × 18 mm), approved for neonatal duct stenting (8). During the inter-stage period controls were performed weekly with ample attention to weight gain and to respiratory rate during baby’s sleep. Outpatient examinations included further peripheral oxygen saturation and blood pressure measurements at all extremities describing systolic and diastolic values and not a simple mean pressure. Echocardiography assessed unrestrictive interatrial communication, tricuspid-valve competence, right ventricular function as well as non-obstructed retrograde perfusion of the hypoplastic aortic arch including cerebral blood flow (Doppler flow of anterior cerebral artery) and the blood-flow pattern of the coeliac trunk. The blood flow through the stented duct was evaluated aiming a systolic Doppler velocity of less than 2.5 m/s. Reducing the diastolic left-to-right shunt to the least possible extent, the infant received a combined treatment:
- Bisoprolol (0.1 mg/kg daily) aiming a heart rate (HR) below 120 beats per minute (bpm);
- Lisinopril (0.1 mg/kg daily) to reduce the systemic vascular resistance (SVR) without jeopardizing a sufficient coronary perfusion pressure and a sufficient systolic/diastolic blood pressure amplitude measured at the right arm, if an aberrant right subclavian artery was excluded;
- Spironolactone (6.25 mg daily) under the hypothesis to influence right ventricle (RV) fibrosis seen in almost all RV single ventricle patients over time.
Referring to the last mentioned three medications this protective strategy is summarized as our pediatric cardiac B-L-S strategy (16). Comprehensive stage II surgery was performed in an age of 4.5 months with a body-weight of 4.8 kg under selective cerebral perfusion by a PTFE-shunt connected at the brachiocephalic trunk together in moderate hypothermia at core body temperature of 26 to 28 °C. Aortic clamping time was 59 minutes, CPB time including reperfusion time was 220 minutes. Intraoperatively moderate bleeding could be controlled, no arrhythmias or further complications occurred. With continuously infused milrinone (0.5–1 µg/kg/min) and low dose norepinephrine (0.05–0.15 µg/kg/min) as well as inhaled nitric oxide (iNO) in a dosage of 20 ppm, the patient was transferred by the anesthesiologic team to the pediatric cardiac intensive care unit (PCICU); at admission, HR was 155 bpm due to a slightly elevated core temperature of 38 °C. Continuously measured blood pressure (IBP) was 75/40 mmHg, upper systemic vein and pulmonary artery pressures (PAP) were 17 mmHg, central venous pressure (CVP) measured by a catheter positioned in the lower caval vein was 10 mmHg, the calculated transpulmonary pressure gradient (TPG) 7 mmHg; cerebral regional oxygen saturation measured by near infrared spectroscopy (NIRS) was 40% and the peripheral oxygen saturation (SaO2) 80%. Considering our post-operative protocol (Figure 1), the still intubated patient was ventilated in an assisted mode on pressure-controlled ventilation with following respirator settings: peak inspiratory pressure (Pinsp) was 19 cmH2O, respiratory rate 16/min, inspiratory time (tinsp) 0.9 s, tidal-volume 11 mL/kg, positive end-expiratory pressure (PEEP) 4 cmH2O, mean airway pressure (MAP) 9 cmH2O, FiO2 0.9, iNO 20 ppm. Hemoglobin concentration (Hb) was 14.3 g/dL, mixed venous saturation (SvO2) 43%. Continuously infused cardiovascular drugs consisted of norepinephrine (0.15 µg/kg/min), milrinone (0.5 µg/kg/min), clonidine (2 µg/kg/h), esmolol (80 µg/kg/min), remifentanil (0.3 µg/kg/min).
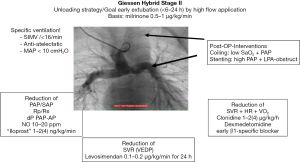
Transthoracic echocardiography showed a mild diastolic dysfunction but good systolic contractility without evidence of pericardial effusion or hemothorax. X-ray by additional application of contrast medium excluded obstruction both of the Glenn-anastomosis as well as the LPA. Patient was actively cooled down to 36.0 °C. In presence of declined microcirculation and cool extremities fresh frozen plasma was administered (30 mL/kg). HR declined to 125 bpm, esmolol and norepinephrine infusions were reduced stepwise. NIRS increased to 55%, SaO2 to 83%, SvO2 to 57%. Continuous infusion of furosemide (6 mg/kg/day) combined with theophylline (5 mg/kg/day) was started in term of a prophylactic measure to avoid or to reduce any significant peripheral edema formation. Remifentanil was stopped; 4 h after admission with adequate alertness and cough strength the patient was extubated and immediately connected to binasal continuous positive airway pressure (CPAP) ventilation (PEEP 6 cmH2O, pressure support set at 5 cmH2O, FiO2 0.85, iNO 20 ppm). Inhalations of diluted norepinephrine were administered every 4 h. In absence of active bleeding anticoagulation with heparin (300 IE/kg/day) was started 6 h postoperatively and continued with targeted active partial thromboplastin time (aPTT) 45–55 s. For the next 12 h on CPAP-support remifentanil was re-started in low dosage of 0.1 µg/kg/min, together with clonidine infusion, the dosage of clonidine was monitored preferably by HR monitoring (aim less than 125 bpm). Continuous norepinephrine infusion was stopped 7 h after admission at intensive care unit (ICU).
On first postoperative day with better alertness and good airway patency CPAP-support was switched to humified high-flow-nasal cannula (flow 15 L/min, FiO2 0.7, iNO 10 ppm). SaO2 was 84%, PAP 14 mmHg, CVP 8 mmHg, correspondent TPG 6 mmHg. With continued infusion of furosemide and theophylline diuresis improved to 4 mL/kg/day, control echocardiography revealed good global ventricular function and only slight pleural effusions without any therapeutic implication. Ultrasound showed preserved bilateral diaphragmatic movement and was unsuggestive of diaphragmatic paralysis. Patient had warm extremities and only subtle eyelid-edema. Remifentanil was ceased and switched to fixed single doses of analgetics. Episodes of agitation were covered with single doses midazolam and chloral hydrate. With improved feeding tolerance at second postoperative day oral anti-congestive therapy with B-L-S was restarted. iNO was stepwise reduced and replaced by oral sildenafil (0.5 mg/kg/6 h). At second postoperative day furosemide was switched to single doses, and oral hydrochlorothiazide (1 mg/kg/12 h) was added. Under prophylactic therapy with cefuroxime, there were neither clinical nor serum chemical signs of infection. Milrinone was finished at third postoperative day. Over the next 4 postoperative days oral feeding was reinstituted, analgesic medications were adapted to need, clonidine was tapered off. After 7 days the patient could be transferred to the open pediatric ward.
Discussion
From 1998 till 2017, 121 patients with HLHS received a Giessen comprehensive stage II surgery (15). The mean age at surgery was 4.5 months, mean bodyweight 5 kg. The mean aortic cross-clamp time was 69 minutes, CPB time including our re-perfusion strategy was 249 minutes. Besides 15 patients, all has been admitted on ICU with closed chest and without significant bleeding (12). Both aspects are prior conditions for an ideal ICU course. The approach outlined above reflects our center experience (8) to optimize oxygen delivery (DO2) by establishing a sufficient cardiac function and by enhancing pulmonary blood flow in face of counteracting factors causing high oxygen consumption (VO2) as tachycardia, increased body temperature, systemic inflammatory response (SIRS), pain and insufficient spontaneous breathing after comprehensive stage II operation.
Close monitoring of HR, SaO2, SAP, PAP, CVP, PaO2, SvO2, NIRS and delta-temperature are primary requirements to detect hemodynamic instability and to guide the therapy. The calculated TPG differentiated between post- or pre-capillary component of an increased pulmonary vascular resistance. Immediate imaging after admission is done by transthoracic echocardiography to assess cardiac function, tricuspid regurgitation and to rule out cardiac tamponade or hemothorax. During further PCICU course, repetitive echocardiographic evaluations are mandatory to control cardiac function and to adapt therapy promptly. As early visualization of Glenn-anastomosis is difficult by echocardiography X-ray “angiography” allows to detect obstructions of the Glenn-anastomosis and the reconstructed PA-branches. Obstructions of the LPA are with an incidence of almost 35% the Achilles heel of the comprehensive stage II (8,13).
Considering the limitations to increase DO2 following comprehensive stage II, circulatory support should focus on minimizing systemic und myocardial VO2. HR control (target value 110–130 bpm) plays a pivotal role for circulatory therapy. Tachycardia that occurs despite sufficient analgosedation can be easily addressed by active cooling to a body temperature of 35.0 to 36.5 °C. In patients with an anergic reaction, in term of vasoplegia due to CPB or analgosedation, norepinephrine can become necessary for maintaining an adequate renal perfusion pressure. As the use of norepinephrine is oftentimes accompanied by tachycardia short-acting betablockers like esmolol or landiolol can rectify this side-effect. Clonidine, a centrally acting alpha2-agonist, unifies diverse desirable effects. It lowers SVR and HR and acts as an analgesic adjuvant. Caution is advised with cumulative effects of clonidine in higher dosage ranges: an occurring relative bradycardia can be easily corrected by pausing continuous infusion for several hours. As clonidine itself also causes withdrawal it should be tapered off. For inotropic support a continuous infusion of milrinone (0.5–1 µg/kg/min) is administered. If necessary levosimendan (0.1 µg/kg/min) can be safely combined to milrinone. Digoxin (loading dose 0.01 mg/kg i.v. every 8 h on day 1, than adapted to serum levels of 0.5–0.8 nmol/L) can be an additional option with favorable effects on HR. Epinephrine is used rarely due to its undesirable effects on HR and VO2. Nevertheless, it can be administered safely provided that a dosage of 0.04 µg/kg/min is not exceeded.
Fluid management for achieving an appropriate cardiac preload is the most challenging part of the circulatory therapy and can be a complex task. The isolated meaning of CVP or HR is very restricted. In a very dynamic postoperative physiology, best guidance is offered by simple clinical examination of peripheral tissue perfusion. Only the combined interpretation of clinical signs and monitoring parameters allows a deliberate fluid therapy. Consequences of fluid mismanagement are not all a cosmetic issue. Over-infusion by liberal fluid management often results in tissue and alveolar edema and in deterioration of cellular oxygen supply and pulmonary oxygen delivery. To prevent this problem a continuous infusion of furosemide is started early and not at a later timepoint. It is combined with a low-dosage continuous application of theophylline due to its diuretic-, anti-inflammatory and anti-obstructive effects.
Ventilation and respiratory support are another two crucial factors in postoperative care of comprehensive stage II patients. The passive pulmonary blood-flow requires an optimized pulmonary vascular resistance. Therefore, emphasis must be placed on an anti-atelectatic, anti-obstructive ventilation preventing intra-pulmonary shunts. Our ventilation regime is targeted to higher tidal-volumes than usually used in lung protective ventilation strategy (10–12 instead 4–6 mL/kg). Given a so recruited lung low respiratory rate and low MAP (<10 cmH2O) facilitate lung perfusion. Given adequate alertness early extubation within the first 4 h after surgery is performed independently of high oxygen supply. Sufficient spontaneous breathing without atelectasis after thoracotomy and CPB seems to be hardly to achieve in infants. However, immediate non-invasive respiratory support prevents alveolar derecruitment and subsequent deterioration of pulmonary blood-flow and reduces work of breathing and thus VO2. Special regard should be paid to patients with a desired interatrial restriction during the inter-stage I; slightly congested lungs tend to bronchial und vascular hyperreagibility and bronchial obstruction after CPB-surgery and can be susceptible to complicating pulmonary infections. Noninvasive respiratory support also enables a liberal analog-sedation during the early postoperative period. From our experience continuous infusion of short-acting remifentanil in combination with clonidine allows the bridge the acute phase of the first postoperative day.
Finally, absence of pulmonary artery thrombosis and diaphragmatic paralysis is even fundamental for an optimal postoperative course.
Within almost 20-year experience we treated more than 154 patients with HLHS and about 90 patients with HLH-complex by Hybrid approach. Considering a learning curve for both, interventional transcatheter (17) as well as surgical techniques [operation on “beating heart” without hypothermic cardiac arrest (18)], the postoperative mortality (within 30 days or prior to hospital discharge) in a cohort of 121 patients with comprehensive stage II was 6.6% (15). Centers in Toronto, Canada (19) or Ohio, USA (14) reported similar numbers. The latter underwent a learning curve and could significantly reduce their mortality from 19% to 4% in comprehensive stage II by adapting protocols.
Conclusions
Description of protocols can facilitate exchange of experiences and improve the learning curve.
From our experience intensive care management in comprehensive stage II should be concentrated on three goals:
- Minimizing systemic and myocardial oxygen consumption;
- Reducing SVR;
- Optimizing pulmonal perfusion and ventricular preload.
Cornerstones of intensive care therapy are early extubation and non-invasive respiratory support, balanced fluid management avoiding excessive edema and economization of HR. Especially in a pathophysiological situation when oxygen delivery cannot be sufficiently increased control of oxygen consumption and avoidance of stress are the most important success parameters.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med 1983;308:23-6. [Crossref] [PubMed]
- Bailey LL, Nehlsen-Cannarella SL, Doroshow RW, et al. Cardiac allotransplantation in newborns as therapy for hypoplastic left heart syndrome. N Engl J Med 1986;315:949-51. [Crossref] [PubMed]
- Dapper F, Bauer J, Kroll J, et al. Clinical experience with heart transplantation in infants. Eur J Cardiothorac Surg 1998;14:1-5; discussion 5-6. [Crossref] [PubMed]
- Gibbs JL, Wren C, Watterson KG, et al. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: a new approach to palliation for the hypoplastic left heart syndrome. Br Heart J 1993;69:551-5. [Crossref] [PubMed]
- Michel-Behnke I, Akinturk H, Schranz D. Fate of the stented arterial duct. Circulation 2000;102:E178. [Crossref] [PubMed]
- Akintuerk H, Michel-Behnke I, Valeske K, et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation 2002;105:1099-103. [Crossref] [PubMed]
- Akinturk H, Michel-Behnke I, Valeske K, et al. Hybrid transcatheter-surgical palliation: basis for univentricular or biventricular repair: the Giessen experience. Pediatr Cardiol 2007;28:79-87. [Crossref] [PubMed]
- Schranz D, Bauer A, Reich B, et al. Fifteen-year single center experience with the "Giessen Hybrid" approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol 2015;36:365-73. [Crossref] [PubMed]
- Reich B, Heye K, Tuura R, et al. Neurodevelopmental Outcome and Health-related Quality of Life in Children With Single-ventricle Heart Disease Before Fontan Procedure. Semin Thorac Cardiovasc Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Heye KN, Knirsch W, Latal B, et al. Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before Fontan completion. Pediatr Res 2018;83:63-70. [Crossref] [PubMed]
- Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol 2005;26:190-9. [Crossref]
- Yerebakan C, Valeske K, Elmontaser H, et al. Hybrid therapy for hypoplastic left heart syndrome: Myth, alternative, or standard? J Thorac Cardiovasc Surg 2016;151:1112-21, 1123.e1-5.
- Ohye RG, Schranz D, D'Udekem Y. Current Therapy for Hypoplastic Left Heart Syndrome and Related Single Ventricle Lesions. Circulation 2016;134:1265-79. [Crossref] [PubMed]
- Galantowicz M, Yates AR. Improved outcomes with the comprehensive stage 2 procedure after an initial hybrid stage 1. J Thorac Cardiovasc Surg 2016;151:424-9. [Crossref] [PubMed]
- Yoruker U, Akinturk H. Giessen Procedure as Comprehensive Stage II Palliation With Aortic Arch Reconstruction After Hybrid Bilateral Pulmonary Artery Banding and Ductal Stenting for Hypoplastic Left Heart Syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2018;21:19-27. [Crossref] [PubMed]
- Schranz D, Voelkel NF. "Nihilism" of chronic heart failure therapy in children and why effective therapy is withheld. Eur J Pediatr 2016;175:445-55. [Crossref] [PubMed]
- Schranz D, Michel-Behnke I. Advances in interventional and hybrid therapy in neonatal congenital heart disease. Semin Fetal Neonatal Med 2013;18:311-21. [Crossref] [PubMed]
- Wozniak G, Dapper F, Zickmann B, et al. Selective Cerebral Perfusion Via Innominate Artery in Aortic Arch Replacement Without Deep Hypothermic Circulatory Arrest. Int J Angiol 1999;8:50-6. [Crossref] [PubMed]
- Baba K, Kotani Y, Chetan D, et al. Hybrid versus Norwood strategies for single-ventricle palliation. Circulation 2012;126:S123-31. [Crossref] [PubMed]


