
Transplantation of the failing Fontan
Introduction
For children with complex congenital heart disease and single ventricle physiology, staged palliation ending in the Fontan procedure is the standard of care. Initially described in 1971 by Francis Fontan with a goal of eliminating cyanosis in a young woman with tricuspid atresia, the Fontan palliation is a total cavopulmonary connection of the inferior and superior vena cavae to the pulmonary arteries, with no subpulmonary ventricle (1). The procedure has greatly improved the short term morbidity and mortality for children with single ventricle heart disease, however Dr. Fontan himself said the procedure imposes a “gradually declining functional capacity and premature late death… the Fontan operation is, therefore, palliative but not curative” (2). In the nearly five decades since Dr. Fontan’s original report of the Fontan palliation, the procedure has undergone several surgical advances in technique with subsequent improvement in patient outcomes (3,4). While most initial reports of Fontan survival were single center reports, several recent studies based on multi-center data have shown significant improvement in previous survival estimates. A 2018 meta-analysis of Fontan patients estimated 20-year Fontan survival at 75–85% (5). Studies looking only at Fontans completed in the modern era are even more optimistic. The Pediatric Heart Network’s 2017 report of their Fontan cohort estimated 12-year survival at 90% (6). Indeed, multiple retrospective single center studies have shown that when Fontan patients are divided into eras, accounting for changes in clinical and surgical practice, that survival improves in the later years (7,8). With this in mind, the number of patients both reaching Fontan as well as living with Fontan is likely to increase in the upcoming decades (9). Although the improvement in recent Fontan survival is certainly impressive, the fact remains that even the most optimistic reports have 10% or more of Fontan patients either dying or requiring heart transplant by early adulthood.
As the population of patients living with Fontan physiology continues to grow, the population is itself also changing. Hypoplastic left heart syndrome is now the most common diagnosis of patients undergoing Fontan completion as the result of surgical advancements as well as increased knowledge in the field of intensive care about perioperative management for the first two stages of palliation (10). Furthermore, registry data has found that patients with hypoplastic left heart syndrome are more likely to develop Fontan failure (11,12), thus possibly increasing need for heart failure management and cardiac transplantation as this population survives longer into adulthood. The purpose of this article is to review the unique indications and challenges of evaluation and peri-operative management of heart transplantation of the growing Fontan population.
Fontan circulation and categories of failure
The indications for heart transplantation in Fontan patients are inherently linked to the consequences of Fontan physiology. The Fontan circulation places the pulmonary circulation in series with the systemic circulation with no subpulmonary ventricle and relies on passive flow through the pulmonary bed resulting in obligatory systemic venous hypertension. Because of the lack of a subpulmonary pumping chamber, the Fontan circulation results in less preload than the two-ventricle circulation and remodeling of the pulmonary venous vasculature (13). Thus, Fontan physiology results in chronic vascular changes both in the systemic venous and pulmonary venous circulations. Each of these physiologic changes may lead to symptomatic intolerance of Fontan physiology, often collectively termed Fontan failure despite the appearance and progression of Fontan failure being heterogenous. To help guide both prognostication and treatment, modalities of Fontan failure are often grouped into phenotypical and mechanistic subtypes (14). For all these phenotypes, failure may progress despite aggressive medical management, and heart transplantation may become the only option for improved quality of life and long-term survival. Of these phenotypes, ventricular failure (50%) and protein losing enteropathy (PLE) (40%) are the top two most common reasons for transplant referral (15,16).
To provide a framework for referral for advanced heart failure consultation, the Advanced Cardiac Therapies Improving Outcomes Network (ACTION), a national collaborative of pediatric heart failure clinicians, researchers, parents, and patients has recently released a reference entitled “Considerations for Advanced Heart Failure Consultation in Fontan Patients”, included in Figure 1 (17). The ACTION collaborative is hopeful that timely referral for advanced heart failure consultation and collaborative care of Fontan patients with heart failure will continue to enhance patient outcomes but the effect of changing referral patterns remains to be seen.

Ventricular dysfunction
Failure of the Fontan circulation can be the result of systolic or diastolic ventricular dysfunction, perhaps more analogous to heart failure in the patient with two-ventricle circulation (14). Chronic effects of single ventricle physiology result in changes to the geometry and function of the ventricle itself, manifesting in abnormal relaxation or contraction of the myocardium or distortion of the systemic atrioventricular valve (18). Despite being a common indication for referral for heart transplantation, there is overall a paucity of published data about ventricular failure in the Fontan population. The Pediatric Heart Network identified that right ventricular morphology was associated with poorer ventricular function (12). It has also been suggested that systolic ventricular failure may impact outcomes in the pediatric Fontan population more than in adults, as systolic ventricular dysfunction is not a predictor of death or heart transplantation listing in the adult Fontan population (19). The impact of diastolic heart failure in the Fontan population is also likely underappreciated, with several studies showing that increased atrial pressures after Fontan are predictive of non-survival (7,20). Better described than either systolic or diastolic dysfunction is the association of atrioventricular valve insufficiency and poor Fontan outcomes. A recent international study found that atrioventricular valve failure was significantly associated with failure of the Fontan circulation (21).
Fontan circuit failure
Fontan failure can also be unique to the Fontan (or pulmonary) circuit itself. Fontan pathway failure clinically manifests as chronic right heart failure with hepatomegaly, ascites, and edema, as well as cyanosis from right-to-left shunts within the pulmonary pathway (14). It has been shown in multiple studies that cyanosis in Fontan patients is associated with death (6), adverse events around procedures (22), and that increased oxygen saturation decreases the risk of a major event (19).
Lymphatic failure
Also related to the Fontan circulation is abnormalities of the lymphatic system. PLE, or leakage of lymph from the intestinal lumen, is an independent risk factor for mortality in the Fontan population (23). The intestinal lymphatics in Fontan patients are likely inherently abnormal, as immune abnormalities in Fontan patients are similar to those found in non-Fontan patients with PLE caused by intestinal or hepatic lymphangiectasia (24). Likewise, plastic bronchitis or leakage of lymph from the bronchial tree is also associated with increased mortality (25). Advances in MRI based lymphatic imaging has identified abnormal pulmonary lymphatic perfusion in Fontan patients with plastic bronchitis (26). Lymphatic failure in Fontan patients is exceptionally challenging from a management perspective not only because of the limited treatment options, but also because many of these patients have seemingly normal hemodynamics (27,28). There appears to be a subset of individuals who develop lymphatic abnormalities that manifest in a variety of ways only partly affected by hemodynamics (29). There is some suspicion that lymphatic failure results not only from abnormalities of the lymphatic tree but also from vascular changes of non-vital organs such as the mesentery, resulting in relative local changes in systemic venous resistance and leakage of lymph (30).
Extra-cardiac organ failure
Even in hemodynamically well compensated Fontan patients, cardiac output is impaired with less ability to increase output during high demand (31), resulting in chronic subacute cardiac insufficiency that may eventually lead to end organ damage particularly affecting the liver and the kidneys. Fontan associated liver disease (FALD) is a known entity secondary to chronic liver congestion in patients after Fontan completion. Pathology studies have shown that most if not all patients after Fontan will have changes on a histological level, with more advanced disease progressing to cirrhosis or even hepatocellular carcinoma (32). The degree of fibrotic change appears to only be associated with the length of time spent as a Fontan patient and not with hemodynamic or other patient or systemic factors, suggesting that the disease is both progressive and directly results from Fontan circulation alone (33). Screening and monitoring for progression of FALD can be very challenging, as even patients with advanced disease tend to be asymptomatic with near normal biochemical and functional hepatic tests (34). While less discussed than FALD, kidney disease in Fontan patients is also an important consideration. Kidney disease is probably under-recognized in patients after Fontan, with several studies showing reduced glomerular filtration rate (GFR) (35), increased risk of glomerular and tubular injury (36), and chronic kidney disease (CKD) stage 2 or greater in 10–25% of patients (37).
Fontan heart transplant outcomes
Early studies of outcome from heart transplant in Fontan patients showed decreased post heart transplant survival in pediatric Fontan patients when compared to other patients undergoing heart transplantation (38-40). Recent studies are more optimistic and suggest that survival for pediatric Fontan patients after heart transplantation is similar to non-Fontan congenital heart disease patients (16,41,42). Pediatric Fontan patients with preserved ventricular function previously were reported to have worse survival compared to patients with impaired ventricular function (43), however even pediatric Fontan patients with preserved ventricular function have had improvement in survival in more recent years (42). Likewise, survival after pediatric heart transplantation as a whole has also improved. (44). Table 1 lists reports from multiple single center and multicenter studies regarding Fontan survival after heart transplantation. Improved survival after heart transplantation for Fontan patients as well as pediatric patients in general is likely multifactorial, secondary to advances in surgical technique, pre- and post-operative care, and immunosuppression as well as possible temporal changes in patient selection. Careful consideration during the pre-listing evaluation and surgical planning may continue to improve post-transplantation outcomes in this population.
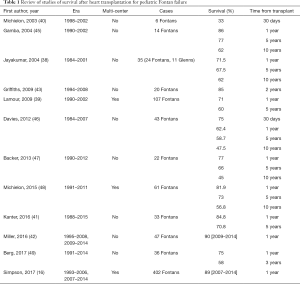
Full table
Risk factors, patient optimization, and post-transplant outcomes
Lymphatic failure
Although a common indication for heart transplantation and frequently discussed as a risk factor for post-transplant outcomes, the diagnosis of PLE alone does not increase wait list or post-transplant mortality (50,51). In addition, although It has been suggested that patients with PLE are relatively immunocompromised (24), a recent study from the Pediatric Heart Transplant study found no difference in rejection or post-transplant infection rates between PLE and non-PLE Fontan patients. In a separate multicenter study, there was no change in wait-list or post-transplant mortality with aggressive treatment of PLE (51). Mortality was also not associated with degree of growth impairment or duration of disease in patients with PLE. However, there was an association between using non-standard immunosuppression in patients with PLE and post-transplant mortality, specifically nonuse of mycophenolate mofetil. Further studies are needed to determine what if any alterations in immunosuppression should be used in this patient population. Of note, multiple studies have noted resolution of PLE after transplantation (38,41,45,52-54). The most recent multi-center report of plastic bronchitis and post-transplantation outcomes showed that plastic bronchitis is a far less common indication for transplantation, and that patients with plastic bronchitis have worse early mortality than controls but no difference in long term outcomes. Similarly to PLE, plastic bronchitis appears to resolve post transplantation (55).
Sensitization
Fontan patients are often human leukocyte antigen sensitized. Multiple prior surgeries result in significant blood product exposure. Homograft material is also often used in vascular reconstruction during the staged palliation and associated with long-term sensitization in half of patients (56). For these reasons, Fontan patients may be more likely to be sensitized prior to transplant, which is known to be associated with decreased 1-year post transplant survival (57,58). A study from the Pediatric Heart Transplant Study in 2006 found that 16.5% of Fontan patients and 12.8% of non-Fontan patients with congenital heart disease had a pre-transplantation panel reactive antibodies (PRA) >20%, compared with only 2.3% of patients without congenital heart disease (52). However, these relatively higher PRAs may not in actuality affect post-transplant outcomes. A recent study described a third of Fontan patients with a PRA greater than 10%, but with no significant differences in outcome (16). More studies are needed to determine if sensitization affects outcomes in this population, and in turn if desensitization would improve outcomes. A small study of highly sensitized patients with congenital heart disease awaiting transplantation found a significant decrease in PRA with intravenous immunoglobulin and rituximab treatment (59). Using decellularized graft material in the initial stages of palliation may also decrease sensitization at time of transplant listing (60).
Age
Timing of listing for Fontan patients must be carefully considered. There are no established criteria for patients with Fontan failure and timing of transplant listing (43,61). Not only current clinical status, but also wait list time and age at listing need to be considered. A recent study showed that patients had a significantly longer wait time when listed after their 18th birthday than those listed before their 18th birthday. While this study did not show any significant difference in waitlist mortality between the two groups, timing of listing for Fontan patients who are medically fragile should be carefully considered (62). The effect of age at listing on outcomes is unclear, with one study showing an increased risk of death in an early age group (39), and another multi-center study of only adult patients showed that Fontan patients who were listed at a younger age had improved mortality over time (63).
Functional status
A recent study has shown that worse functional status prior to transplant is associated with worse short and long term outcomes (64). Therefore, for Fontan patients prior to transplant exercise and mobility should be emphasized and improved upon if safe and possible. Fontan patients have at baseline a decreased VO2 from predicted and show a decline of VO2 over time (65). It is also well documented that a substantial decline in VO2 over time is a predictor of mortality or need for heart transplantation in Fontan patients (66,67). This may not only potentially improve post-transplant outcomes but also may improve heart failure symptoms in general in this population. There is a growing body of literature that exercise, especially focusing on lower body resistance training, can improve VO2 and clinical status in Fontan patients. Resistance muscle training has been shown to improve cardiac filling, stroke volume, and exercise capacity in adult Fontan patients (68). Referral to cardiac rehabilitation or physical therapy as appropriate should be considered for all Fontan patients being considered for heart transplantation.
Nutrition
Multiple studies have suggested that nutrition and growth are risk factors for perioperative heart transplant outcomes in the congenital heart disease population. Poor nutrition has been shown to increase wait list mortality in patients with congenital heart disease (69). Likewise, poor growth is a risk factor for worse outcomes in congenital heart disease patients following heart transplant (70). There is likely an opportunity to further improve outcomes by optimizing nutrition in Fontan patients prior to transplant. This may have increased importance in patients with chronic protein losses, like Fontan patients with PLE.
Non-cardiac end organ failure
End organ failure in Fontan patients is prevalent and is a risk factor for need for heart transplantation (71,72). Of particular interest is FALD and its role in timing of heart transplant listing. Despite its initial subclinical nature, FALD can complicate Fontan failure and may be significant enough to be a contraindication to heart-only transplantation (34). Progressive Fontan failure symptoms with progressive liver dysfunction may be a trigger earlier consideration for heart transplantation prior to further deterioration of the liver, however these patients may not meet more urgent listing criteria and thus may have a longer wait list time. Those with more advanced disease may require heart-liver transplantation, although the exact indications for heart-liver transplant in this population is not yet well understood (73). A single-center study showed no difference in 1-year post transplant survival for Fontan patients with or without cirrhosis (74), and thus even more advanced disease may not be a complete contraindication to heart-only transplantation. Furthermore although several case series from single centers report good outcomes heart-liver transplantations in single ventricle patients with end state liver disease (75,76), more information is needed to determine which patients truly require both organs.
Likewise, kidney function should be carefully considered in the transplant evaluation. Pre-operative renal failure is known to be a predictor of worse outcomes in all patients post heart transplant (77), and similarly pre-transplant renal failure is a strong predictor of early mortality in Fontan patients after heart transplantation (46). Similar to liver failure, advanced kidney disease may be a relative contraindication for heart only transplantation, and heart-kidney transplantation may be a consideration for some patients. Monitoring of kidney function in patients with Fontan failure is key to identifying patients prior to development of advanced kidney failure.
Mechanical ventilation
Ventilator support has been consistently associated with increased mortality after heart transplantation both for patients with and without Fontan palliation (15,52,61,78). Mechanical ventilation in Fontan patients, like other patients with complex congenital heart disease, may be related to abnormalities of pulmonary vasculature or lung parenchyma, lung or diaphragm injury, or airway abnormalities (79). It could also be secondary to cardiac insufficiency and Fontan failure and be an indication of worse clinical status (15). Attempts should be made to optimize cardiac status and remove the patient from invasive mechanical ventilation; this both reduces the independent risk and also allows for improved rehabilitation and other optimization. Evaluation and listing for transplantation prior to need for mechanical ventilation will likely continue to improve post-transplant outcomes (52).
Ventricular assist devices (VADs)
The VAD experience in the single ventricle population is growing. While most of the published literature is case reports, it would appear that some Fontan patients may benefit from use of VADs prior to transplantation. These patients are primarily those with systolic dysfunction, diastolic dysfunction, or both as their mechanism of failure (80,81). There may also be a role for biventricular support devices in patients with very high central venous pressures to improve end-organ function, transplant candidacy, and post-transplant outcomes (82,83). Because of the prevalence of aorto-pulmonary collaterals in Fontan patients, it is generally accepted that continuous flow assist devices are preferable to account for the changes in inflow in the presence of these vessels (80). A recent review of case reports of VADs in Fontan patients had fourteen out of nineteen successful cases of VAD therapy (bridged to transplant, bridged to recovery, or still supported) (80). Another single center series of all single ventricle patients with assist devices reported only four Fontan patients with only one surviving to transplant (84). Finally, the Berlin Heart registry lists three out of five patients with Fontan who survived to transplant (85). While the risks of VAD must be considered prior to using mechanical support in this patient population, some Fontan patients may benefit from VAD bridge to transplant to mitigate severity of illness and improve upon some of the above risk factors including avoidance of mechanical ventilation, end-organ damage and optimization of functional status and nutrition. Collaboration across centers, like through the ACTION group as previously mentioned, will allow increased knowledge about assist devices in this patient population.
Catheter based intervention of aorto-pulmonary collaterals
Evaluating for and intervening on collateral vessels is also important in the pre-transplant optimization process. Collateral vessels are common in Fontan patients and increase the risk of bleeding as well as potentially affect post-operative hemodynamics. Patients with single ventricle physiology are known to develop aorto-pulmonary collateral vessels which can lead to recirculation and increased volume load on the systemic ventricle or the new graft (86). Aggressive pre-heart transplant embolization of aortopulmonary collateral vessels increases systemic flow pre-transplant (87) and may decrease bleeding post-transplant. Additionally, the presence of aortopulmonary collaterals post-transplant may lead to prolonged inotropic need and inadequate cardiac output despite good graft function (42,88).
Surgical risk
Transplantation of the Fontan patient presents surgical challenges unique to this patient population (46,89). Fontan patients have several surgical risk factors for worse mortality after heart transplantation including multiple previous sternotomies and longer ischemic times due to the need for Fontan takedown and pulmonary artery reconstruction at the time of their transplant (39,44). Using donor ischemic time and bypass time as surrogates for operative complexity, a single center study showed that Fontan patients have more complex transplant operations than controls (41). Multiple studies have shown that when compared to other patients with congenital heart disease, Fontan patients are more likely to require pulmonary artery reconstruction and have longer cardiopulmonary bypass times at time of heart transplantation (38,46,47,90). Pulmonary artery reconstruction has previously been identified as a risk factor for worse outcomes after heart transplantation, although this finding included all congenital heart disease and was not specific to Fontan (91). All of these surgical risk factors also contribute to risk of post-operative hemorrhage, which has been identified as a cause of early mortality for Fontan patients post-transplantation especially in the early era (38,92). Surgical planning prior to transplantation may minimize some surgical risks. Multiple single center reports have included pre-operative cross sectional imaging to better definite intrathoracic relationships and anatomy to decrease intraoperative hemorrhage as well as minimize cardiac bypass time and decrease warm ischemic time during reconstruction (42).
Post-operative risk: intensive care management
Post-operatively although transplanted Fontan patients no longer have Fontan physiology, vascular changes from longstanding Fontan circulation may impact post-operative care. There is data that some failing Fontan patients have decreased systemic vascular resistance with preserved cardiac index (93). Likewise, markers of decreased endothelial function correlate with decreasing exercise capacity in Fontan patients (94) and thus many Fontan patients at time of transplant will have abnormalities of their systemic vascular function. These patients are likely at increased risk for abnormal vascular tone post-operatively after heart transplantation and use of close hemodynamic monitoring, inotropic support, and extra-corporal membrane oxygenation should be used as necessary. Vasoplegia syndrome, or persistent low systemic vascular resistance despite multiple intravenous pressors at high dose, is associated with high morbidity and mortality after heart transplantation in adults (95,96). Beyond factors unique to Fontan patients that cause vascular dysfunction previously noted, Fontan patients also have multiple operative and medical risk factors for vasoplegia syndrome including potentially long cardiopulmonary bypass time due to the need for reconstruction at the time of transplant and aspirin use (95). Attention to vasoplegia management post heart transplantation has been identified as a potential reason for improved outcomes in the modern era (42). In addition to traditional vasoactive infusions used to manage post-operative vasoplegia, there may be a role for extra-corporeal membrane oxygenation (ECMO) support. The authors’ own institution has had good anecdotal success with transient ECMO support in refractory vasoplegia following cardiac transplantation; in the last 6 years, 3 out of 4 Fontan patients requiring post-transplant ECMO have had >1-year survival.
Abnormalities of the pulmonary vasculature may also affect post-operative care. Autopsy studies have shown that Fontan patients have adverse pulmonary vascular remodeling, with the degree of changes correlating with time since Fontan completion (97). Additional studies have shown that patients farther from their Fontan completion have evidence of elevated transpulmonary gradients post heart transplantation (98). While Fontan patients may not meet diagnostic criteria for pulmonary hypertension, aggressive use of pulmonary vasodilators post-transplant should be considered.
Finally, given the many surgical risks for post-operative hemorrhage including repeat sternotomies, relative coagulopathy, and vascular reconstruction, bleeding should be carefully monitored for and blood products should be given and coagulopathy corrected appropriately.
Conclusions
The population of patients living with Fontan physiology is increasing with more patients reaching Fontan completion and patients with Fontan living longer. Ultimately, many of these patients will develop Fontan failure and require heart transplantation. Survival for Fontan patients after heart transplantation is improving in the modern era, likely due to increased knowledge about risk factors affecting pre and post-operative management. Fontan patients have unique challenges in the medical management peri-heart transplantation including high prevalence of liver and kidney disease, sensitization, relative immunosuppression and increased risk of infection, vasoplegia and increased pulmonary vascular resistance. Surgical challenges include complex pulmonary artery reconstructions, long cardiac bypass times, and increased risk of hemorrhage. The need exists for increasing collaboration via multicenter efforts as well as national and international Fontan registry data to increase knowledge of risk factors for Fontan failure, for morbidity and mortality around heart transplantation, and to overall improve outcomes for this growing community of unique patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971;26:240-8. [Crossref] [PubMed]
- Fontan F, Kirklin JW, Fernandez G, et al. Outcome after a "perfect" Fontan operation. Circulation 1990;81:1520-36. [Crossref] [PubMed]
- d'Udekem Y, Iyengar AJ, Cochrane AD, et al. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation 2007;116:I157-64. [Crossref] [PubMed]
- Stewart RD, Pasquali SK, Jacobs JP, et al. Contemporary Fontan operation: association between early outcome and type of cavopulmonary connection. Ann Thorac Surg 2012;93:1254-60; discussion 1261. [Crossref] [PubMed]
- Poh CL, d'Udekem Y. Life After Surviving Fontan Surgery: A Meta-Analysis of the Incidence and Predictors of Late Death. Heart Lung Circ 2018;27:552-9. [Crossref] [PubMed]
- Atz AM, Zak V, Mahony L, et al. Longitudinal Outcomes of Patients With Single Ventricle After the Fontan Procedure. J Am Coll Cardiol 2017;69:2735-44. [Crossref] [PubMed]
- Pundi KN, Johnson JN, Dearani JA, et al. 40-Year Follow-Up After the Fontan Operation: Long-Term Outcomes of 1,052 Patients. J Am Coll Cardiol 2015;66:1700-10. [Crossref] [PubMed]
- Rogers LS, Glatz AC, Ravishankar C, et al. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol 2012;60:1018-25. [Crossref] [PubMed]
- Schilling C, Dalziel K, Nunn R, et al. The Fontan epidemic: Population projections from the Australia and New Zealand Fontan Registry. Int J Cardiol 2016;219:14-9. [Crossref] [PubMed]
- Jacobs JP, Maruszewski B. Functionally univentricular heart and the fontan operation: lessons learned about patterns of practice and outcomes from the congenital heart surgery databases of the European association for cardio-thoracic surgery and the society of thoracic surgeons. World J Pediatr Congenit Heart Surg 2013;4:349-55. [Crossref] [PubMed]
- Iyengar AJ, Winlaw DS, Galati JC, et al. The extracardiac conduit Fontan procedure in Australia and New Zealand: hypoplastic left heart syndrome predicts worse early and late outcomes. Eur J Cardiothorac Surg 2014;46:465-73; discussion 473. [Crossref] [PubMed]
- Anderson PA, Sleeper LA, Mahony L, et al. Contemporary outcomes after the Fontan procedure: a Pediatric Heart Network multicenter study. J Am Coll Cardiol 2008;52:85-98. [Crossref] [PubMed]
- Khambadkone S, Li J, de Leval MR, et al. Basal pulmonary vascular resistance and nitric oxide responsiveness late after Fontan-type operation. Circulation 2003;107:3204-8. [Crossref] [PubMed]
- Book WM, Gerardin J, Saraf A, et al. Clinical Phenotypes of Fontan Failure: Implications for Management. Congenit Heart Dis 2016;11:296-308. [Crossref] [PubMed]
- Schumacher KR, Almond C, Singh TP, et al. Predicting graft loss by 1 year in pediatric heart transplantation candidates: an analysis of the Pediatric Heart Transplant Study database. Circulation 2015;131:890-8. [Crossref] [PubMed]
- Simpson KE, Pruitt E, Kirklin JK, et al. Fontan Patient Survival After Pediatric Heart Transplantation Has Improved in the Current Era. Ann Thorac Surg 2017;103:1315-20. [Crossref] [PubMed]
- Schumacher KR, Kindel SJ. Considerations for advanced heart failure consultation in Fontan Patients: Guidance for primary cardiologists. In: Advanced Cardtiac Therapies Improving Outcomes Network. Available online: http://www.actionlearningnetwork.org. Accessed May 16 2019.
- Rychik J, Goldberg DJ. Late consequences of the Fontan operation. Circulation 2014;130:1525-8. [Crossref] [PubMed]
- Elder RW, McCabe NM, Veledar E, et al. Risk factors for major adverse events late after Fontan palliation. Congenit Heart Dis 2015;10:159-68. [Crossref] [PubMed]
- Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85-92. [Crossref] [PubMed]
- King G, Ayer J, Celermajer D, et al. Atrioventricular Valve Failure in Fontan Palliation. J Am Coll Cardiol 2019;73:810-22. [Crossref] [PubMed]
- Egbe AC, Khan AR, Ammash NM, et al. Predictors of procedural complications in adult Fontan patients undergoing non-cardiac procedures. Heart 2017;103:1813-20. [Crossref] [PubMed]
- John AS, Johnson JA, Khan M, et al. Clinical outcomes and improved survival in patients with protein-losing enteropathy after the Fontan operation. J Am Coll Cardiol 2014;64:54-62. [Crossref] [PubMed]
- Magdo HS, Stillwell TL, Greenhawt MJ, et al. Immune Abnormalities in Fontan Protein-Losing Enteropathy: A Case-Control Study. J Pediatr 2015;167:331-7. [Crossref] [PubMed]
- Schumacher KR, Singh TP, Kuebler J, et al. Risk factors and outcome of Fontan-associated plastic bronchitis: a case-control study. J Am Heart Assoc 2014;3:e000865. [Crossref] [PubMed]
- Dori Y, Keller MS, Rome JJ, et al. Percutaneous Lymphatic Embolization of Abnormal Pulmonary Lymphatic Flow as Treatment of Plastic Bronchitis in Patients With Congenital Heart Disease. Circulation 2016;133:1160-70. [Crossref] [PubMed]
- Sarkola T, Jaeggi E, Slorach C, et al. Assessment of vascular remodeling after the Fontan procedure using a novel very high resolution ultrasound method: arterial wall thinning and venous thickening in late follow-up. Heart Vessels 2013;28:66-75. [Crossref] [PubMed]
- Lambert E, d'Udekem Y, Cheung M, et al. Sympathetic and vascular dysfunction in adult patients with Fontan circulation. Int J Cardiol 2013;167:1333-8. [Crossref] [PubMed]
- Mertens L, Hagler DJ, Sauer U, et al. Protein-losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg 1998;115:1063-73. [Crossref] [PubMed]
- Rychik J, Gui-Yang S. Relation of mesenteric vascular resistance after Fontan operation and protein-losing enteropathy. Am J Cardiol 2002;90:672-4. [Crossref] [PubMed]
- Gewillig M, Brown SC, Eyskens B, et al. The Fontan circulation: who controls cardiac output? Interact Cardiovasc Thorac Surg 2010;10:428-33. [Crossref] [PubMed]
- Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: Chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg 2005;129:1348-52. [Crossref] [PubMed]
- Goldberg DJ, Surrey LF, Glatz AC, et al. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J Am Heart Assoc 2017. [Crossref] [PubMed]
- Daniels CJ, Bradley EA, Landzberg MJ, et al. Fontan-Associated Liver Disease: Proceedings from the American College of Cardiology Stakeholders Meeting, October 1 to 2, 2015, Washington DC. J Am Coll Cardiol 2017;70:3173-94. [Crossref] [PubMed]
- Sharma S, Ruebner RL, Furth SL, et al. Assessment of Kidney Function in Survivors Following Fontan Palliation. Congenit Heart Dis 2016;11:630-6. [Crossref] [PubMed]
- Opotowsky AR, Baraona FR, Mc Causland FR, et al. Estimated glomerular filtration rate and urine biomarkers in patients with single-ventricle Fontan circulation. Heart 2017;103:434-42. [Crossref] [PubMed]
- Lee D, Levin A, Kiess M, et al. Chronic kidney damage in the adult Fontan population. Int J Cardiol 2018;257:62-6. [Crossref] [PubMed]
- Jayakumar KA, Addonizio LJ, Kichuk-Chrisant MR, et al. Cardiac transplantation after the Fontan or Glenn procedure. J Am Coll Cardiol 2004;44:2065-72. [Crossref] [PubMed]
- Lamour JM, Kanter KR, Naftel DC, et al. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol 2009;54:160-5. [Crossref] [PubMed]
- Michielon G, Parisi F, Squitieri C, et al. Orthotopic heart transplantation for congenital heart disease: an alternative for high-risk fontan candidates? Circulation 2003;108 Suppl 1:II140-9. [Crossref] [PubMed]
- Kanter KR. Heart Transplantation in Children after a Fontan Procedure: Better than People Think. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2016;19:44-9. [Crossref] [PubMed]
- Miller JR, Simpson KE, Epstein DJ, et al. Improved survival after heart transplant for failed Fontan patients with preserved ventricular function. J Heart Lung Transplant 2016;35:877-83. [Crossref] [PubMed]
- Griffiths ER, Kaza AK, Wyler von Ballmoos MC, et al. Evaluating failing Fontans for heart transplantation: predictors of death. Ann Thorac Surg 2009;88:558-63; discussion 563-4. [Crossref] [PubMed]
- Dipchand AI, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: seventeenth official pediatric heart transplantation report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:985-95. [Crossref] [PubMed]
- Gamba A, Merlo M, Fiocchi R, et al. Heart transplantation in patients with previous Fontan operations. J Thorac Cardiovasc Surg 2004;127:555-62. [Crossref] [PubMed]
- Davies RR, Sorabella RA, Yang J, et al. Outcomes after transplantation for "failed" Fontan: a single-institution experience. J Thorac Cardiovasc Surg 2012;143:1183-92.e4. [Crossref] [PubMed]
- Backer CL, Russell HM, Pahl E, et al. Heart transplantation for the failing Fontan. Ann Thorac Surg 2013;96:1413-9. [Crossref] [PubMed]
- Michielon G, van Melle JP, Wolff D, et al. Favourable mid-term outcome after heart transplantation for late Fontan failure. Eur J Cardiothorac Surg 2015;47:665-71. [Crossref] [PubMed]
- Berg CJ, Bauer BS, Hageman A, et al. Mortality Risk Stratification in Fontan Patients Who Underwent Heart Transplantation. Am J Cardiol 2017;119:1675-9. [Crossref] [PubMed]
- Schumacher KR, Gossett J, Guleserian K, et al. Fontan-associated protein-losing enteropathy and heart transplant: A Pediatric Heart Transplant Study analysis. J Heart Lung Transplant 2015;34:1169-76. [Crossref] [PubMed]
- Schumacher KR, Yu S, Butts R, et al. Fontan-associated protein-losing enteropathy and postheart transplant outcomes: A multicenter study. J Heart Lung Transplant 2019;38:17-25. [Crossref] [PubMed]
- Bernstein D, Naftel D, Chin C, et al. Outcome of listing for cardiac transplantation for failed Fontan: a multi-institutional study. Circulation 2006;114:273-80. [Crossref] [PubMed]
- Brancaccio G, Carotti A, D'Argenio P, et al. Protein-losing enteropathy after Fontan surgery: resolution after cardiac transplantation. J Heart Lung Transplant 2003;22:484-6. [Crossref] [PubMed]
- Holmgren D, Berggren H, Wahlander H, et al. Reversal of protein-losing enteropathy in a child with Fontan circulation is correlated with central venous pressure after heart transplantation. Pediatr Transplant 2001;5:135-7. [Crossref] [PubMed]
- Gossett JG, Almond CS, Kirk R, et al. Outcomes of cardiac transplantation in single-ventricle patients with plastic bronchitis: a multicenter study. J Am Coll Cardiol 2013;61:985-6. [Crossref] [PubMed]
- O'Connor MJ, Lind C, Tang X, et al. Persistence of anti-human leukocyte antibodies in congenital heart disease late after surgery using allografts and whole blood. J Heart Lung Transplant 2013;32:390-7. [Crossref] [PubMed]
- Jacobs JP, Quintessenza JA, Boucek RJ, et al. Pediatric cardiac transplantation in children with high panel reactive antibody. Ann Thorac Surg 2004;78:1703-9. [Crossref] [PubMed]
- Kaufman BD, Shaddy RE. Immunologic considerations in heart transplantation for congenital heart disease. Curr Cardiol Rev 2011;7:67-71. [Crossref] [PubMed]
- Schumacher KR, Ramon DS, Kamoun M, et al. HLA desensitization in pediatric heart transplant candidates: efficacy of rituximab and IVIg. J Heart Lung Transplant 2012;31:1041-2. [Crossref] [PubMed]
- Lim HM, Friedland-Little J, Yu S, et al. Use of Decellularized Cryopreserved Allografts During Single Ventricle Reconstruction Results in Lower HLA Sensitization Than Standard Allograft. J Heart Lung Transplant 2017;36:S77-8. [Crossref]
- Kovach JR, Naftel DC, Pearce FB, et al. Comparison of risk factors and outcomes for pediatric patients listed for heart transplantation after bidirectional Glenn and after Fontan: an analysis from the Pediatric Heart Transplant Study. J Heart Lung Transplant 2012;31:133-9. [Crossref] [PubMed]
- Peng DM, Qu Q, McDonald N, et al. Impact of the 18th birthday on waitlist outcomes among young adults listed for heart transplant: A regression discontinuity analysis. J Heart Lung Transplant 2017;36:1185-91. [Crossref] [PubMed]
- Karamlou T, Diggs BS, Welke K, et al. Impact of single-ventricle physiology on death after heart transplantation in adults with congenital heart disease. Ann Thorac Surg 2012;94:1281-7; discussion 1287-8. [Crossref] [PubMed]
- Ravi Y, Stock E, Lella SK, et al. Does Functional Status at Listing and Time of Heart Transplant Influence Survival in Heart Transplant Recipients? J Heart Lung Transplant 2017;36:S179. [Crossref]
- Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008;52:99-107. [Crossref] [PubMed]
- Giardini A, Hager A, Pace Napoleone C, et al. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg 2008;85:818-21. [Crossref] [PubMed]
- Cunningham JW, Nathan AS, Rhodes J, et al. Decline in peak oxygen consumption over time predicts death or transplantation in adults with a Fontan circulation. Am Heart J 2017;189:184-92. [Crossref] [PubMed]
- Cordina RL, O'Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol 2013;168:780-8. [Crossref] [PubMed]
- Godown J, Friedland-Little JM, Gajarski RJ, et al. Abnormal nutrition affects waitlist mortality in infants awaiting heart transplant. J Heart Lung Transplant 2014;33:235-40. [Crossref] [PubMed]
- Castleberry C, White-Williams C, Naftel D, et al. Hypoalbuminemia and poor growth predict worse outcomes in pediatric heart transplant recipients. Pediatr Transplant 2014;18:280-7. [Crossref] [PubMed]
- Assenza GE, Graham DA, Landzberg MJ, et al. MELD-XI score and cardiac mortality or transplantation in patients after Fontan surgery. Heart 2013;99:491-6. [Crossref] [PubMed]
- Wilson TG, d'Udekem Y, Winlaw DS, et al. Hepatic and renal end-organ damage in the Fontan circulation: A report from the Australian and New Zealand Fontan Registry. Int J Cardiol 2018;273:100-7. [Crossref] [PubMed]
- Greenway SC, Crossland DS, Hudson M, et al. Fontan-associated liver disease: Implications for heart transplantation. J Heart Lung Transplant 2016;35:26-33. [Crossref] [PubMed]
- Simpson KE, Esmaeeli A, Khanna G, et al. Liver cirrhosis in Fontan patients does not affect 1-year post-heart transplant mortality or markers of liver function. J Heart Lung Transplant 2014;33:170-7. [Crossref] [PubMed]
- Hollander SA, Reinhartz O, Maeda K, et al. Intermediate-term outcomes after combined heart-liver transplantation in children with a univentricular heart. J Heart Lung Transplant 2013;32:368-70. [Crossref] [PubMed]
- Vaikunth SS, Concepcion W, Daugherty T, et al. Short-term outcomes of en bloc combined heart and liver transplantation in the failing Fontan. Clin Transplant 2019.e13540. [PubMed]
- Odim J, Wheat J, Laks H, et al. Peri-operative renal function and outcome after orthotopic heart transplantation. J Heart Lung Transplant 2006;25:162-6. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. [Crossref] [PubMed]
- Healy F, Hanna BD, Zinman R. Pulmonary complications of congenital heart disease. Paediatr Respir Rev 2012;13:10-5. [Crossref] [PubMed]
- Carlo WF, Villa CR, Lal AK, et al. Ventricular assist device use in single ventricle congenital heart disease. Pediatr Transplant 2017. [Crossref] [PubMed]
- Giridharan GA, Ising M, Sobieski MA, et al. Cavopulmonary assist for the failing Fontan circulation: impact of ventricular function on mechanical support strategy. ASAIO J 2014;60:707-15. [Crossref] [PubMed]
- Valeske K, Yerebakan C, Mueller M, et al. Urgent implantation of the Berlin Heart Excor biventricular assist device as a total artificial heart in a patient with single ventricle circulation. J Thorac Cardiovasc Surg 2014;147:1712-4. [Crossref] [PubMed]
- Nathan M, Baird C, Fynn-Thompson F, et al. Successful implantation of a Berlin heart biventricular assist device in a failing single ventricle. J Thorac Cardiovasc Surg 2006;131:1407-8. [Crossref] [PubMed]
- Poh CL, Chiletti R, Zannino D, et al. Ventricular assist device support in patients with single ventricles: the Melbourne experience. Interact Cardiovasc Thorac Surg 2017;25:310-6. [Crossref] [PubMed]
- Weinstein S, Bello R, Pizarro C, et al. The use of the Berlin Heart EXCOR in patients with functional single ventricle. J Thorac Cardiovasc Surg 2014;147:697-704; discussion 704-5. [Crossref] [PubMed]
- Whitehead KK, Gillespie MJ, Harris MA, et al. Noninvasive quantification of systemic-to-pulmonary collateral flow: a major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging 2009;2:405-11. [Crossref] [PubMed]
- Dori Y, Glatz AC, Hanna BD, et al. Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: a pilot study. Circ Cardiovasc Interv 2013;6:101-6. [Crossref] [PubMed]
- Krishnan US, Lamour JM, Hsu DT, et al. Management of aortopulmonary collaterals in children following cardiac transplantation for complex congenital heart disease. J Heart Lung Transplant 2004;23:564-9. [Crossref] [PubMed]
- Jacobs JP, Quintessenza JA, Chai PJ, et al. Rescue cardiac transplantation for failing staged palliation in patients with hypoplastic left heart syndrome. Cardiol Young 2006;16:556-62. [Crossref] [PubMed]
- Carey JA, Hamilton JR, Hilton CJ, et al. Orthotopic cardiac transplantation for the failing Fontan circulation. Eur J Cardiothorac Surg 1998;14:7-13; discussion 13-4. [Crossref] [PubMed]
- Chen JM, Davies RR, Mital SR, et al. Trends and outcomes in transplantation for complex congenital heart disease: 1984 to 2004. Ann Thorac Surg 2004;78:1352-61; discussion 1352-61. [Crossref] [PubMed]
- Michielon G, Parisi F, Di Carlo D, et al. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg 2003;24:502-10; discussion 510. [Crossref] [PubMed]
- Hebson CL, McCabe NM, Elder RW, et al. Hemodynamic phenotype of the failing Fontan in an adult population. Am J Cardiol 2013;112:1943-7. [Crossref] [PubMed]
- Goldstein BH, Urbina EM, Khoury PR, et al. Endothelial Function and Arterial Stiffness Relate to Functional Outcomes in Adolescent and Young Adult Fontan Survivors. J Am Heart Assoc 2016;5. [Crossref] [PubMed]
- Patarroyo M, Simbaqueba C, Shrestha K, et al. Pre-operative risk factors and clinical outcomes associated with vasoplegia in recipients of orthotopic heart transplantation in the contemporary era. J Heart Lung Transplant 2012;31:282-7. [Crossref] [PubMed]
- Byrne JG, Leacche M, Paul S, et al. Risk factors and outcomes for 'vasoplegia syndrome' following cardiac transplantation. Eur J Cardiothorac Surg 2004;25:327-32. [Crossref] [PubMed]
- Ridderbos FJ, Wolff D, Timmer A, et al. Adverse pulmonary vascular remodeling in the Fontan circulation. J Heart Lung Transplant 2015;34:404-13. [Crossref] [PubMed]
- Mitchell MB, Campbell DN, Ivy D, et al. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg 2004;128:693-702. [Crossref] [PubMed]


