Unusual clinical and histologic findings in a child with mixed dentition with hereditary gingival fibromatosis: a case report
Introduction
Hereditary gingival fibromatosis (HGF), also known as familial or idiopathic gingival fibromatosis, is a rare oral disease characterized clinically by a benign, fibrous and slowly progressive enlargement of the keratinized gingiva (1). HGF may appear as an isolated condition or coexist with a genetic syndrome or disease, such as hypertrichosis (2), progressive hearing loss (3), mental retardation (4) and generalized aggressive periodontitis (5). It has been reported that HGF can be caused by mutation of the SOS1 gene, which encodes a guanine nucleotide exchange factor for ras proteins, but mutations in other genes are also likely involved (6). Additionally, HGF is known to be transmitted as a Mendelian trait in an autosomal dominant or, less commonly, an autosomal recessive mode (1).
Oral manifestations of HGF affect the masticatory mucosa in both dental arches, but it does not spread beyond the muco-gingival junction (7). The degree of enlargement may vary from mild to severe and the gingival enlargement is of normal color, firm and non-hemorrhagic. The excess gingival tissue can result in functional difficulties, drifting of teeth, prolonged retention of primary dentition, poor plaque control, and may also cause psychological and esthetic problems (8).
HGF typically manifests with the onset of permanent dentition, but also can occur with the eruption of the primary teeth and rarely is present at birth (9). In this paper we report a rare three-generation HGF case and describe the clinical and histopathology features of the proband in the mixed dentition, which may help us learn more about the process of HGF and confirm that gingival inflammation is closely related to the gingival overgrowth of HGF. Besides, the opportunity choice on the surgical intervention and recurrence risk of HGF are discussed. We present the following case inaccordance with the CARE-Guideline (10).
Medical history
An 8-year-old boy, was referred to the Department of Oral and Maxillofacial Surgery, Fifth Affiliated Hospital of Sun Yat-sen University by his parents, with a chief complaint of gingival overgrowth causing impaired appearance. The mother stated that his problem began at the age of 4 years old and the condition had become increasingly severe with the emergence of the permanent incisors. He never sought treatment or took any medication that could be associated with gingival overgrowth, such as cyclosporine or calcium channel blockers. The past history of the patient was not contributory to oral findings.
Family history
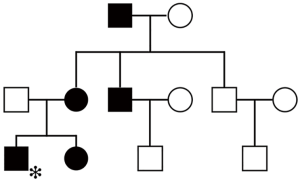
Among the three latest generations of the proband’s kindred, his sister, mother, maternal grandfather, and one of his two uncles had a similar gingival condition (Figure 1). Besides, his sister’s problem began at the age of 4 years old, too. The tissue enlargement of his mother was milder than his and was surgically treated approximately 20 years ago (Figure 2). These data provide evidence that HGF was transmitted by an autosomal dominant trait in the present family. Syndromic abnormalities commonly seen in association with HGF, such as hypertrichosis and mental retardation, were not observed in the family.
Examination
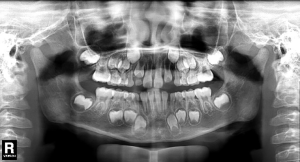
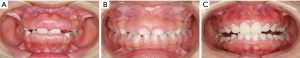
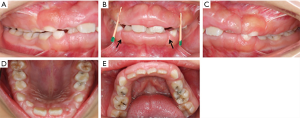
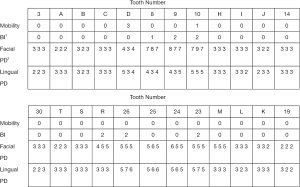
Intraoral examination showed moderate to severe gingival overgrowth involving both labial and lingual regions in the maxillary and mandibular anterior areas. The enlarged tissue was firm, pink, and remarkably, the clear dividing lines which deep into the bottom of labial gingiva between permanent incisors and deciduous teeth were observed (teeth 7 had, in fact, almost reached the position but was covered by fibrous gingival tissue and retained deciduous tooth) (Figure 3). Periodontal probing revealed mild gingivitis but alveolar bone resorption was not found (Figure 4). Besides, panoramic radiograph showed normal alveolar bone (Figure 5).
Diagnosis and treatment
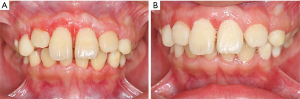
The patient’s manifestations and inheritance trait showed a typical HGF characteristic. As the gingival tissue increased, eating dysfunctions, especially impaired appearance caused severe damage to physical and mental health which made the patient and his parents urged for surgical intervention. The initial phase of periodontal treatment, which comprised scaling and oral hygiene instruction was taken. Surgery which consisted of gingivectomy and gingivoplasty technique in both labial and lingual regions in the maxillary and mandibular anterior areas was performed. The retained maxillary deciduous lateral incisor (#D) was extracted during surgery. After the surgery, the patient was given a prescription for an antibiotic and an analgesic and a periodontal dressing was placed for 1 week before removal. No pain or discomfort was complained and no infection was found throughout the observation period. The gingiva recovered well when the patient returned for observation 2 weeks later and the upper left incisor (#7) erupted to the occlusal surface when he came back 7 weeks after surgery (Figure 6). We had repeatedly stressed the importance of maintaining oral hygiene to prevent recurrence. The patient didn’t demonstrate any recurrence of gingival overgrowth over a 1-year follow-up period and the debris index-simplified (DI-S) is 0−1 at each review. The most significant effect of surgery was a cosmetic improvement. After the operation, my son loved to laugh more and brushed his teeth more carefully than before said the mother of the proband.
Histopathology
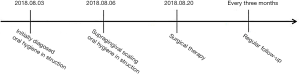
At the time of the surgery, biopsies were obtained from the enlarged tissue and stained with hematoxylin and eosin (H&E). The epithelium typically structured with elongated rete pegs penetrated deep into the connective tissue were observed (Figure 7). Microscopic evaluation of the sections from permanent incisors area revealed that dense, mature parallel collagen fiber bundles dominated the deep connective tissue while sections from deciduous canine area showed collagen bundles running in all directions (Figure 7). Additionally, severe epithelial hyperplasia and numerous blood vessels were noted in specimens with intense inflammation (Figure 7), mainly lymphocytes and plasma cells infiltration, whereas in tissue with mild inflammation showed only long and deep epithelial papillae and moderate amount of blood vessels. Additionally, the main procedures and operations of this episode of care was organized as a timeline (Figure 8).

Discussion
According to the pedigree chart, HGF was transmitted by an autosomal dominant mode in the present three-generation family. Genetic studies demonstrated that HGF could be caused by mutation of the SOS1 gene, however, researches on three Chinese families (11) and two Polish families (12) with HGF did not detect any mutation in SOS1. Notably, the latest research revealed RE1-silencing transcription factor (REST) final-exon-truncating mutations caused HGF in three unrelated families (13). Thus, HGF may involve several genes and more studies are needed to probe the HGF-related mutations.
Increasing efforts have been made to understand the genetic and molecular basis of the gingival enlargement of HGF since it was first reported in 1856. However, the pathogenic mechanism of the disease remains largely unknown. It was pointed out that the presence of teeth seemed to be necessary for HGF to occur because the enlargement was most intense during the eruption of both primary and permanent teeth and the condition disappeared or receded with the loss of the teeth (14). The proband described here showed a unusual gingiva condition in the mixed dentition stage which the degree of gingival hyperplasia in permanent incisors area was more severe than that in deciduous teeth area and the clear dividing lines which deep into the bottom of labial gingiva between the two regions were observed. The appearance of this fissure might be related to the acceleration of gingival hyperplasia during the eruption of permanent teeth which further confirmed previous reports. As for histologic features of HGF gingival enlargement, most attentions have been focused on the connective tissue alterations. It is generally accepted that the expansion of gingival tissue in HGF results mostly from the accumulation of excess extracellular matrix (ECM), mainly collagen, and diminished ECM degradation. Studies demonstrated that fibroblasts from HGF produce 30% to 50% more collagen than normal gingival fibroblasts and Type I collagen was the major collagen type produced by HGF (15,16). We observed that the collagen bundles in the permanent incisors area of enlarged tissue were prevalently significantly bulkier than that of the deciduous teeth area and the arrangement was much more regular. The difference might be related to the acceleration of gingival hyperplasia.
HGF cannot be cured but may be controlled with varying degrees of success. Although researchers have suggested that biological agents such as the chimeric IgG variant which can inhibit extracellular collagen fibril formation may be a new tool for the treatment of HGF (17), surgical intervention is still the only way to treat severe gingival hyperplasia. However, the patient still has to deal with the risk of recurrence. It has been demonstrated that recurrence is faster in specimens with dental plaque accumulation, while meticulous plaque control can prevent or minimize recurrence (18,19). However, Emerson (20) reported that the enlargement did not appear to be related to the oral hygiene. In this study, severe epithelial hyperplasia and numerous blood vessels were noted in specimens with intense inflammatory cell infiltration. It was reported that epithelial cells enabled motile and transitioning cells to invade through basement membranes and migrated into connective tissue stroma where they contributed to new ECM production (21). Additionally, Martelli-Junior et al. (22) demonstrated that epithelial hyperplasia was closely related to the gingival overgrowth of HGF. Combining these studies with our findings, we believe that dental plaque accumulation and bacterial infection which leads to severe epithelial hyperplasia is crucial to the gingival overgrowth of HGF. However, the histologic results from the single case might not be applicable to all patients and more clinical studies are required.
The suggested time of surgery is when all of the permanent teeth have erupted because the risk of recurrence is higher if done earlier. However, recurrence is unpredictable. Our research reveals that plaque accumulation caused by gingival hyperplasia in turn aggravates the enlargement, thus forming a vicious circle and eventually leading to various complications. Therefore, we suggest that the surgical treatment should be performed in these patients once they are unable to maintain proper oral hygiene.
Conclusions
To the best of the authors’ knowledge, this is the first case report of gingiva features in HGF patient in the mixed dentition, which further confirms that the eruption of permanent teeth can accelerate gingival hyperplasia. Additionally, it is confirmed that the gingival overgrowth of HGF is closely related to the presence of inflammation in the gingival tissue.
Acknowledgments
Funding: The work was supported by Natural Science Foundation of Guangdong Province, China (No. 2018A030313759); Science Foundation of SMU (No. PY2017N036); Medical Scientific Research Foundation of Guangdong Province, China (No. A2018358).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
References
- Häkkinen L, Csiszar A. Hereditary gingival fibromatosis: characteristics and novel putative pathogenic mechanisms. J Dent Res 2007;86:25-34. [Crossref] [PubMed]
- Horning GM, Fisher JG, Barker BF, et al. Gingival fibromatosis with hypertrichosis. A case report. J Periodontol 1985;56:344-7. [Crossref] [PubMed]
- Hartsfield JK, Bixler D, Hazen RH. Gingival fibromatosis with sensorineural hearing loss: An autosomal dominant trait. Am J Med Genet 1985;22:623-7. [Crossref] [PubMed]
- Araiche M, Brode H. A case of fibromatosis gingivae. Oral Surg Oral Med Oral Pathol 1959;12:1307-10. [Crossref] [PubMed]
- Casavecchia P, Uzel MI, Kantarci A, et al. Hereditary Gingival Fibromatosis Associated With Generalized Aggressive Periodontitis: A Case Report. J Periodontol 2004;75:770-8. [Crossref] [PubMed]
- Hart TC, Zhang Y, Gorry MC, et al. A mutation in the SOS1 gene causes hereditary gingival fibromatosis type 1. Am J Hum Genet 2002;70:943-54. [Crossref] [PubMed]
- Gawron K, Łazarz-Bartyzel K, Potempa J, et al. Gingival fibromatosis: clinical, molecular and therapeutic issues. Orphanet J Rare Dis 2016;11:9. [Crossref] [PubMed]
- Doufexi A, Mina M, Ioannidou E. Gingival overgrowth in children: epidemiology, pathogenesis, and complications. A literature review. J Periodontol 2005;76:3-10. [Crossref] [PubMed]
- Breen GH, Addante R, Black CC. Early onset of hereditary gingival fibromatosis in a 28-month-old. Pediatr Dent 2009;31:286-8. [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Ma Y, Sun Z, Hu Y, et al. Non-syndromic hereditary gingival fibromatosis in three chinese families is not due to SOS1 gene mutations. Cell Biochem Biophys 2014;70:1869-73. [Crossref] [PubMed]
- Gawron K, Bereta G, Nowakowska Z, et al. Analysis of mutations in the SOS-1 gene in two Polish families with hereditary gingival fibromatosis. Oral Dis 2017;23:983-89. [Crossref] [PubMed]
- Bayram Y, White JJ, Elcioglu N, et al. REST Final-Exon-Truncating Mutations Cause Hereditary Gingival Fibromatosis. Am J Hum Genet 2017;101:149-56. [Crossref] [PubMed]
- Coletta RD, Graner E. Hereditary gingival fibromatosis: a systematic review. J Periodontol 2006;77:753-64. [Crossref] [PubMed]
- Coletta RD, Almeida OP, Ferreira LR, et al. Increase in expression of Hsp47 and collagen in hereditary gingival fibromatosis is modulated by stress and terminal procollagen N-propeptides. Connect Tissue Res 1999;40:237-49. [Crossref] [PubMed]
- Martelli-Junior H, Cotrim P, Graner E, et al. Effect of transforming growth factor-beta1, interleukin-6, and interferon-gamma on the expression of type I collagen, heat shock protein 47, matrix metalloproteinase (MMP)-1 and MMP-2 by fibroblasts from normal gingiva and hereditary gingival fibromatosis. J Periodontol 2003;74:296-306. [Crossref] [PubMed]
- Gawron K, Lazarz-Bartyzel K, Lazarz M, et al. In vitro testing the potential of a novel chimeric IgG variant for inhibiting collagen fibrils formation in recurrent hereditary gingival fibromatosis: chimeric antibody in a gingival model. J Physiol Pharmacol 2014;65:585-91. [PubMed]
- Kavvadia K, Pepelassi E, Alexandridis C, et al. Gingival fibromatosis and significant tooth eruption delay in an 11-year-old male: a 30-month follow-up. Int J Paediatr Dent 2005;15:294-302. [Crossref] [PubMed]
- Bansal A, Narang S, Sowmya K, et al. Treatment and two-year follow-up of a patient with hereditary gingival fibromatosis. J Indian Soc Periodontol 2011;15:406-9. [Crossref] [PubMed]
- Emerson TG. Hereditary gingival hyperplasia: A family pedigree of four generations. Oral Surg Oral Med Oral Pathol 1965;19:1-9. [Crossref] [PubMed]
- Zavadil J, Böttinger EP. TGF-β and epithelial-to-mesenchymal transitions. Oncogene 2005;24:5764. [Crossref] [PubMed]
- Martelli-Junior H, Lemos DP, Silva CO, et al. Hereditary gingival fibromatosis: report of a five-generation family using cellular proliferation analysis. J Periodontol 2005;76:2299-305. [Crossref] [PubMed]