The best pharmaceuticals for children—what can we do?
Introduction
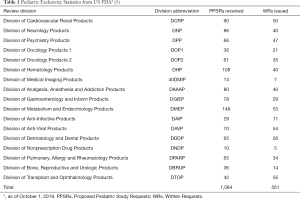
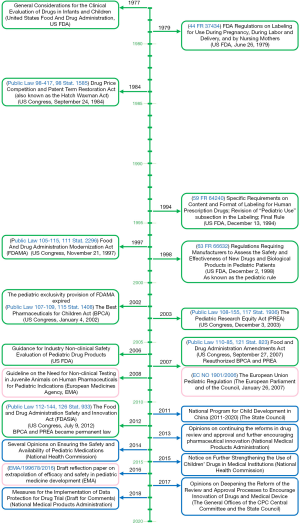
The lack of proper pediatric labeling of medications results in many tragedies, such as Emily, a delightful 6-year-old girl who failed the treatment with imiquimod 5% cream (1). It is very important to legislate to promote the research and development of pediatric drugs. The rules for promoting drug development for children could date back to 1977—The General Considerations for the Clinical Evaluation of Drugs in Infants and Children (Figure 1). To improve the labeling of medications for children, the US Food and Drug Administration (FDA) and Congress promulgated Pediatric Rule in 1994. In 1997, a 6-month extension to existing marketing exclusivity, as known as pediatric exclusivity, which could translate into an about $500 million additional revenue for each drug, was first promulgated in the section 111 of the FDA Modernization Act (FDAMA) if pharmaceutical companies finished the study of medications in pediatric populations (2,3). In 2002, the Best Pharmaceuticals for Children Act (BPCA) was enacted by the US FDA. Followed by its companion legislation acts, the Pediatric Research Equity Act (PREA) in 2003. Both Acts were made permanent in 2012 (Figure 1). The BPCA and the PREA have facilitated the clinical studies in children. Clinical trials conducted under BPCA mainly focused on pediatric-specific or rare diseases. Drug company perform BPCA clinical trials is completely voluntary. But a reward known as Pediatric Exclusivity will be granted by the US FDA with an additional 6 months of marketing exclusivity if the pediatric clinical trials are completed. The BPCA and the PERA have increased labeling of drugs in children. Results from Avant et al. indicated that from September 2007 to September 2016, there were 292 BPCA pediatric therapeutic trials resulted in 107 pediatric labeling changes and 57% pediatric labeling changes had a new or expanded age group (4). New pediatric information has been added into labeling of more than 650 products as a result of BPCA and PREA (4). Based on data from US FDA pediatric exclusivity statistics, till October 1, 2019, there were 1,084 Proposed Pediatric Study Requests (PPSRs) received and 561 Written Requests issued under pediatric exclusivity (Table 1) (5). In the European Union (EU), the Pediatric Regulation came into force in 2007, has promoted the development of pediatric drugs in the EU (Figure 1) (6,7). Anti-infective, gastrointestinal, psychiatry, neurology and cardiovascular are five most commonly studied therapeutic categories (4). However, the US was the most common host country with an 87% of all documented clinical trials sites by country, followed by Canada, the United Kingdom, Russia, South Africa, France and 66 other countries (4). Timeline of key laws, regulations and guidelines to improve pediatric clinical trials are shown in Figure 1. There is still a lack of Acts like BPCA and PREA in China.
Challenges in pediatric clinical trials
It is a small number of children who required treatment with medications when compared to adults. Based on the physiologic changes of pediatric patients, children under 18-year-old are divided into 7 groups by the Pediatric Biopharmaceutics Classification System, which are neonates (≤40 weeks post conception), infants (two groups, 0–6 months old and 6–12 months), toddlers (1–3 years old), children (two groups, 4–6 and 7–12 years old) and adolescents (13–18 years old) (8). The technical complexity of certain types of studies [pharmacokinetic (PK) studies or pharmacodynamic (PD) studies] does increase in younger infants. Real-world data (RWD) could inform pediatric clinical trial design or safety of drugs used in pediatric populations, but there were still some key challenges of using RWD to improve pediatric drug development (9). First and for most, the source of RWD is always not easy to obtain or available for free. Secondly, RWD may presented in an unstructured format made it not easy to understand or effective to use. Transformation of these data into useful information is another big challenge. The last but not the least, the quality of RWD can be challenged by many situations, resulted in omissions or inaccuracies. Despite these limitations, RWD and evidence-based data are still a good way to address children’s incomplete clinical data (10).
Ethical for pediatric clinical trials
Pediatric clinical trials may expand the knowledge of childhood disease, efficacy and safety of treatments. But the balance between providing the children access to benefits and protecting them from potential harms should be fully considered. Ethical considerations are very important for the participation of children in clinical trials. Parents typically provide informed consent for their children to participate in clinical research and children over 7 years old should provide assent about it (11). Not only the ethics committees have the responsibility to ensure clinical trials are appropriate, but the sponsors and investigators should also consider the potential benefits and harms.
Future consideration
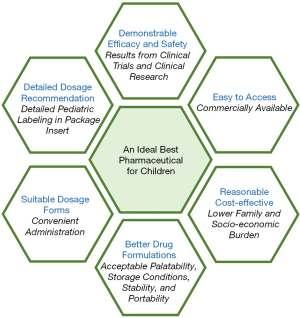
Significant strides have been made with pediatric drug research during the last several decades. Great progress has been made in pediatric pharmaceuticals under the BPCA and PREA (12). Based on pediatric patient organizations, there is still lack of the best pharmaceutics for children in “real-world”. The next challenge will be to make “old drugs” (unpatented, over patented or generic drugs) to be the best pharmaceutics for pediatric therapeutics, and to use framework for prioritization of new drugs development in pediatric specific diseases. The Pediatric Trials Network (PTN) worked under the BPCA to provide the FDA with information to inform label changes for pediatric medicines. Till December, 2019, there were more than 7,000 children enrolled in the 38 PTN studies including 26 clinical trials (13). More than 70 drugs were studied and 21 products with data were submitted to the FDA (13). An evaluation system to confirm what and which is the best pharmaceutic for children is urgent needed. There are six bullet points need to be considered (Figure 2).
Consideration of demonstrable efficacy and safety
Both efficacy and safety are important factor for pediatric populations. Use of off-label and unlicensed medicines are highly associated with adverse drug events in children (14). The relative paucity of efficacy data in pediatric populations may result in continuously exposure to medications that may not derive any therapeutic benefit. Despite some side effects may not be occurred during these medications therapies, huge payments would continue be paid by children’s families, hospital and the health care system for medications that may not be effective. Because of the small number of children who required treatment compared to adults, there are not enough patients to perform a placebo controlled randomized clinical trial to evaluate the efficacy and safety of candidate agents. In this case, RWD and evidence-base data might be a good way at present.
Consideration of dosage recommendations for children
Inappropriate dose selection may result in failed pediatric clinical trials. Dose-finding studies according to age-appropriate dosing strategies should be performed before efficacy and safety studies (15). From neonates to adults, children are in a dynamic state of growth. Drug response and toxicity for children with different age groups are significantly different (3). Dosage recommendations for children should not be a “small adult dose recommendation”. It should be clearly stated in package inserts. However, PK studies and PD studies to evaluate the efficacy and safety of drugs in pediatric populations are very difficult to conduct (16). One important reason for it is physiological changes of children are not a linear process, which lead to PK and PD differences between two different age groups. The existence of priors or difficulties associated with extrapolation from biomarker effect to clinical efficacy or extrapolation from short- to long-term effect are also important reason for not performing pediatric PK/PD study. “Off-label” use of drugs remains a common issue for pediatric patients. The current emphasis of mid-income countries or underdeveloped countries is to find an efficacious and safe dose of older generic drugs in children through RWD or evidence-based data. Statistical modelling and simulation for population-PK are good ways to obtain information for dose selection and dose prediction in pediatrics (16). More importantly, in order to avoid expose children to unnecessary trials and lower the regulatory requirements, the European Medicines Agency (EMA) established an extrapolation framework to create an easier authorization pathway for pediatric drugs development (17). Extrapolation framework is also an important way to make off-label determinations, especially in the therapeutic areas of pediatrics (18).
Consideration of dosage forms for children
Dosage forms are very important for children, especially in neonates, infants and young children. The liquid, suspension and powder medicines are easy to administrate in children, but tablets and capsules are not suitable for neonates and infants. Results from a randomized cross-over trials performed by van Riet-Nales et al. indicated that toddlers and children aged from 1 to 4 years old and their parents preferred small tablet and syrup over the suspension and the suspension over the powder (19). Interestingly, inconsistent with traditional cognition which oral liquid dosage forms such as suspensions and syrups are considered as the favorable types of dosage form to children, many evidences indicated small-sized tablets were the best accepted dosage forms, or at least as good as the syrup (19,20). Despite the World Health Organization (WHO) Model Formulary for Children recommends medicines for children should be provide as a flexible, solid, oral dosage forms, a good designed randomized clinical study is necessary in order to obtain the real preferences of dosage forms among pediatric populations.
Consideration of better drug formulations for children
Acceptable palatability of oral pharmaceuticals is important to facilitate children’s adherence of medicines. Taste is not only the primary motivator for food intake, but also an important motivator for drug intake among children. The preference and acceptability of different dosage forms are also related to taste (21). Children are more sensitive to taste especially to small changes in the sugar content than adults (22). Higher levels of fructose are always preferred among children (22). Because of different individual may have different taste, evaluating an acceptable taste for every child, especially in different regions or countries is difficult (23). Poorly tasting pharmaceuticals may result in “playing hide and seek” when administrate these drugs to pediatric patients (24). Poor taste masking techniques, sweeteners and excipients are utilized in pediatric pharmaceutical development to improve the palatability.
Consideration of cost-effective of pharmaceuticals for children
Lack of financial incentives and low expectation of significant market sales to justify the costs of clinical trials result in a huge gap between clinical trials and pediatric patients. Extended market exclusivity is a method performed by US FDA. However, it may result in additional cost to individuals, hospitals, and medical insurance system. In developing or underdeveloped countries, the market share of patented drugs is relatively small. The same extension of exclusivity regulation is not applied to these countries. To enhance pediatric clinical trials, as Dr. Needle suggested, a pharmaceutical sponsor could donate to a nonprofit organization or academic community to fund pediatric research (25). Based on the consultation with regulatory authorities such as the FDA, sponsors and the academic community could elect which drugs are the most priority needs or most widely used with pediatric patients. In addition, the research of formulation development and some pre-clinical studies can also be handed over to the academia by a pharmaceutical sponsor or the government. The commercial transformation will be carried out after the technology matures gradually. After the completion of the pediatric clinical research, a financial reward or policy support should be given to the pharmaceutical sponsor.
Consideration of drugs accessibility for children
FDA-approval status of medications is of great concerned by children’s parents (26). Mothers and parents aged or order than 45 years old are more likely to prefer FDA-approved medications for their child’s medications (26). However, most parents generally are not aware of all the information contained or lacking in the label. There are many FDA approved products which are either devoid of pediatric labeling or have incomplete pediatric information. But the lack of FDA indications should not limit pediatric patients access to appropriate treatment (27). Recently, some older drugs have been developed into pediatric suitable dosage forms in US, such as spironolactone liquid and diazepam nasal spray, but these pharmaceuticals are not commercially available in China (28). Since 2015, several documents were endorsed to improve drug approval process and better distribution of pediatric-friendly medicines (29). Further efforts are still needed to improve drugs accessibility for children, especially for the best pharmaceuticals for children.
Conclusions
In order to promote the development of the best pharmaceuticals for children, there are six aspects should be focused, which are demonstrable efficacy and safety, detailed dosage recommendation, suitable dosage forms, better drug formulations, reasonable cost-effective and easy to access. Future legislation to enhance pediatric clinical trials or clinical research should expand on these real-world needs of pharmaceuticals for children.
Acknowledgments
Funding: This article is supported by the Fundamental Research Funds for the Central Universities (No. 17JCYB11), the Scientific Research Program of China Hospital Development Institute in Shanghai Jiao Tong University (CHDI-2019-B-17).
Footnote
Conflicts of Interest: HL serves as the unpaid section editor of Translational Pediatrics from Oct 2019 to Sep 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Katz KA, Swetman GL. Imiquimod, molluscum, and the need for a better "best pharmaceuticals for children" act. Pediatrics 2013;132:1-3. [Crossref] [PubMed]
- Cooper KJ. Pediatric marketing exclusivity--as altered by the Best Pharmaceuticals for Children Act of 2002. Food Drug Law J 2002;57:519-44. [PubMed]
- Mazer-Amirshahi M, van den Anker J. Best pharmaceuticals for children: how far have we come? Curr Ther Res Clin Exp 2014;76:32-3. [Crossref] [PubMed]
- Avant D, Wharton GT, Murphy D. Characteristics and Changes of Pediatric Therapeutic Trials under the Best Pharmaceuticals for Children Act. J Pediatr 2018;192:8-12. [Crossref] [PubMed]
- FDA. Pediatric Exclusivity Statistics. FDA. Available online: https://www.fda.gov/drugs/development-resources/pediatric-exclusivity-statistics. 2019. Accessed February 16, 2020 2020.
- Nordenmalm S, Tomasi P, Pallidis C. More medicines for children: impact of the EU paediatric regulation. Arch Dis Child 2018;103:557-64. [Crossref] [PubMed]
- Thabet Y, Slavkova M, Breitkreutz J. 10 years EU regulation of pediatric medicines - impact on cardiovascular drug formulations. Expert Opin Drug Deliv 2018;15:261-70. [Crossref] [PubMed]
- Abdel-Rahman SM, Amidon GL, Kaul A, et al. Summary of the National Institute of Child Health and Human Development-best pharmaceuticals for Children Act Pediatric Formulation Initiatives Workshop-Pediatric Biopharmaceutics Classification System Working Group. Clin Ther 2012;34:S11-24. [Crossref] [PubMed]
- Mulugeta LY, Yao L, Mould D, et al. Leveraging Big Data in Pediatric Development Programs: Proceedings From the 2016 American College of Clinical Pharmacology Annual Meeting Symposium. Clin Pharmacol Ther 2018;104:81-7. [Crossref] [PubMed]
- Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-World Evidence - What Is It and What Can It Tell Us? N Engl J Med 2016;375:2293-7. [Crossref] [PubMed]
- Ott MA, Crawley FP, Saez-Llorens X, et al. Ethical Considerations for the Participation of Children of Minor Parents in Clinical Trials. Paediatr Drugs 2018;20:215-22. [Crossref] [PubMed]
- Ren Z, Zajicek A. Review of the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act: What can the obstetric community learn from the pediatric experience? Semin Perinatol 2015;39:530-1. [Crossref] [PubMed]
- Pediatric Trials Network. Available online: https://pediatrictrials.org/. 2019. Accessed February 17, 2020.
- Hoon D, Taylor MT, Kapadia P, et al. Trends in Off-Label Drug Use in Ambulatory Settings: 2006-2015. Pediatrics 2019. [Crossref] [PubMed]
- Vinks AA, Emoto C, Fukuda T. Modeling and simulation in pediatric drug therapy: Application of pharmacometrics to define the right dose for children. Clin Pharmacol Ther 2015;98:298-308. [Crossref] [PubMed]
- Mahmood I. Dosing in children: a critical review of the pharmacokinetic allometric scaling and modelling approaches in paediatric drug development and clinical settings. Clin Pharmacokinet 2014;53:327-46. [Crossref] [PubMed]
- Stefanska AM, Distlerová D, Musaus J, et al. Extrapolation in the development of paediatric medicines: examples from approvals for biological treatments for paediatric chronic immune-mediated inflammatory diseases. Arch Dis Child 2017;102:952-7. [Crossref] [PubMed]
- Li E, Lobaina E. Application of the FDA Biosimilar Extrapolation Framework to Make Off-Label Determinations. J Manag Care Spec Pharm 2017;23:1227-32. [Crossref] [PubMed]
- van Riet-Nales DA, de Neef BJ, Schobben AF, et al. Acceptability of different oral formulations in infants and preschool children. Arch Dis Child 2013;98:725-31. [Crossref] [PubMed]
- Spomer N, Klingmann V, Stoltenberg I, et al. Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch Dis Child 2012;97:283-6. [Crossref] [PubMed]
- Mennella JA, Roberts KM, Mathew PS, et al. Children's perceptions about medicines: individual differences and taste. BMC Pediatr 2015;15:130. [Crossref] [PubMed]
- Mennella JA, Colquhoun TA, Bobowski NK, et al. Farm to Sensory Lab: Taste of Blueberry Fruit by Children and Adults. J Food Sci 2017;82:1713-9. [Crossref] [PubMed]
- Mistry P, Batchelor H. Evidence of acceptability of oral paediatric medicines: a review. J Pharm Pharmacol 2017;69:361-76. [Crossref] [PubMed]
- Walsh J, Cram A, Woertz K, et al. Playing hide and seek with poorly tasting paediatric medicines: do not forget the excipients. Adv Drug Deliv Rev 2014;73:14-33. [Crossref] [PubMed]
- Needle MN. A proposed modification to the Best Pharmaceuticals for Children Act to benefit pediatric oncology. Pediatr Blood Cancer 2012;59:3-4. [Crossref] [PubMed]
- Yoon EY, Clark SJ, Butchart A, et al. Parental preferences for FDA-approved medications prescribed for their children. Clin Pediatr (Phila) 2011;50:208-14. [Crossref] [PubMed]
- Albrecht J, Adamson AS, Barbieri JS, et al. Lack of a US Food and Drug Administration indication should not limit access to appropriate treatment. J Am Acad Dermatol 2019;80:577-8. [Crossref] [PubMed]
- Meyers R. A Wish List for Drug Development in Pediatrics. J Pharm Sci 2020;109:939-43. [Crossref] [PubMed]
- Wu W, Tang Z, Chen J, et al. Pediatric drug development in China: Reforms and challenges. Pharmacol Res 2019;148:104412. [Crossref] [PubMed]