Reliability and validity of the simplified Chinese version of the Early Onset Scoliosis-24-Item Questionnaire (EOSQ-24)
IntroductionOther Section
Early-onset scoliosis (EOS) is defined as scoliosis first diagnosed in children before 10 years of age and may be secondary to congenital, neuromuscular, syndromic, or idiopathic causes. The large growth potential of the spine in these young children can result in severe deformities and medical comorbidities, including thoracic insufficiency and pulmonary compromise that, if left untreated, may negatively impact their life expectancy (1) and cause significant perturbations to their health-related quality of life (HRQoL) (2). A variety of surgical and non-surgical techniques have been applied to the treatment of EOS, to improve both the natural history of the disease and the HRQoL of the patients. However, a best strategy for its management is still controversial in most cases. It is very important, therefore, to understand the impact of the disease and the effect of different treatment modalities on these patients and their caregivers. While radiologic measurements have been the focus of quantitative assessment for disease progression in the past, increasing emphasis is being placed on utilizing patient-reported outcome measures to incorporate estimates of their physical and psychosocial function (3,4) into a comprehensive evaluation of their health status.
The current form of the English version of EOSQ-24 is a 24-item caregiver questionnaire (5) that was developed following standard processes and specifically for children with EOS, and was modified from its original form with 33 items (6). In order to facilitate global collaboration in research efforts and compare research study results across populations, standardized HRQoL assessment tools are needed. This is especially true for rare conditions like EOS, where the world’s data may be collectively used to improve care. Therefore, it is important to translate and culturally adapt established instruments, such as the EOSQ-24, into different languages, following standard protocols (7). Previous studies have shown reliability and validity for the Turkish and Spanish versions of EOSQ-24 (8,9). In this study, we aimed to translate and culturally adapt the English version of EOSQ-24 into simplified Chinese following established international guidelines, and to test for its reliability and validity among EOS patients and their parents in mainland China. In addition, this represented an effort to further increase international awareness of the need for standardized evaluation of the HRQoL aspects of children with EOS and their caregivers. Simplified Chinese is the contemporary Chinese primarily used in mainland China, Singapore, and Malaysia, and is different from the traditional Chinese routinely used in Taiwan, Hong Kong and Macau. A traditional Chinese version of EOSQ-24 was recently published (10), however, previous studies on the Scoliosis Research Society-22 Patient Questionnaire (SRS-22) and the Spinal Appearance Questionnaire (SAQ) demonstrated the necessity for both traditional (11,12) and simplified Chinese versions (13,14) of the same instruments to better address the issues of specificity and accuracy in HRQoL evaluation among different subgroups of patients. We present the following article in accordance with the SURGE reporting checklist (available at http://dx.doi.org/10.21037/tp-19-177).
MethodsOther Section
Study design
Permission was obtained from the original authors of EOSQ-24 to develop and validate the instrument in simplified Chinese. The study took place from February 2015 to December 2015 and included two stages: (I) translation and cultural adaptation of the English version of EOSQ-24 into simplified Chinese, and (II) testing for reliability and validity in a sample of consecutive post-treatment patients with Mandarin-speaking parents from mainland China recruited during their follow-up visits in the clinic. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Peking University Third Hospital (No. M2015115) and informed consent was taken from all the patients. A sample size of 60 or more would likely provide sufficient power based on estimations from similar studies published previously (8-10).
Translation and cross-cultural adaptation
The English version of EOSQ-24 was first independently forward-translated by two native speakers of Chinese who were fluent in English, with one of them having no medical background. The two versions of simplified Chinese translation were compared and any discrepancies were discussed by an expert panel consisting of two pediatric spine surgeons, one rehabilitation specialist, one psychologist, and the two translators until consensus was reached. The consensus version was then backward-translated into English separately by two native English-speakers who had a good command of Chinese and who were naïve to the design of the study and had no access to the English version of the instrument. The translations were then reviewed by the expert panel and the back-translators for semantic, idiomatic, and conceptual equivalency (15) between the simplified Chinese and the English versions of EOSQ-24. Issues of ambiguities were resolved and a final version was approved. Pre-final testing with semi-structured interviews was performed by a research assistant on parents of 15 post-treatment patients and a Likert scale was used during the interviews. The process was in accordance with the guideline recommended by Beaton et al. (7).
The EOSQ-24
The EOSQ-24 has become the primary outcome measure for HRQoL among children with EOS. It contains 24 items and represents a subjective assessment based on parent-report. It comprises of 11 domains: general health, pain, pulmonary function, mobility, physical function, daily living, fatigue, emotion, parental burden, financial burden, and satisfaction (6). Items are scored between 1 and 5, with higher scores indicating better function. Domain scores and total scores in the simplified Chinese version were calculated in the same way as in the English version of EOSQ-24, and were reported as a score between 0 and 100.
Data quality
A dedicated research assistant was responsible for ascertaining completion of all questionnaire items and to answer any questions that the participants might have. Response distribution for each item and of each domain was assessed by their mean, standard deviation and median. The floor and ceiling effects as determined by the percentage of participants with the minimum and maximum possible scores were calculated, and in general, floor and ceiling effects of less than 30% were considered acceptable (16). Inter-item correlations were calculated to establish item internal consistency.
Reliability
Cronbach’s α quantifies the degree of homogeneity among different items within a questionnaire and a value between 0.7 and 0.95 generally indicates excellent internal consistency (17). A low Cronbach’s α suggests low correlation between items supposed to measure the same construct. “Cronbach’s α if item deleted” was calculated to verify the inter-relatedness of each individual item with the other items and each domain with the other domains in the simplified Chinese version of EOSQ-24.
Discriminative validity
We hypothesized that the simplified Chinese version of EOSQ-24 was capable of differentiating patients based on their demographic and clinical characteristics. The impact of several potential factors was examined. Correlation analyses were performed between the total questionnaire scores and continuous variables using the Spearman’s correlation coefficient test. No assumptions for normal distribution of the EOSQ-24 scores were assumed. For comparison of the average total questionnaire scores of different patient categories, the Mann-Whitney U-test was used if there were two groups and the Kruskal-Wallis test was used if there were three or more groups. Bonferroni corrections were applied for multiple comparisons. The level of significance was set at 0.05 a priori.
Statistical analysis
All statistical analyses were performed and all plot illustrations were generated using the Statistical Package for the Social Sciences (SPSS 20.0, Chicago, IL, USA) software.
ResultsOther Section
Subject characteristics
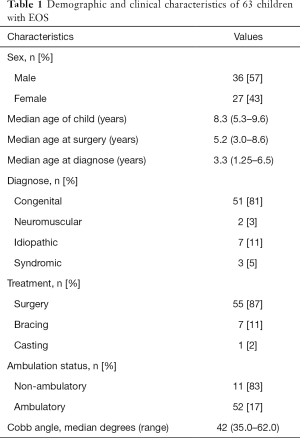
Parents of a heterogeneous group of 63 consecutive children with EOS of different etiologies and at different treatment stages and their parents participated in this study. All completed the questionnaire with no missing items. Demographic and clinical characteristics of the patients were summarized in Table 1.
Full table
Translation and cross-cultural adaptation
During pilot-testing, parents were found to have difficulty understanding the phrase “shortness of breath” and how it might interfere with their child’s function. Necessary changes in wording and addition of further explanation were made after discussion with the parents to improve comprehensibility while at the same time ensuring it carried the same meaning as in the original version. No additional modification was considered necessary based on parent queries regarding each questionnaire item and their corresponding answer choices.
Data quality
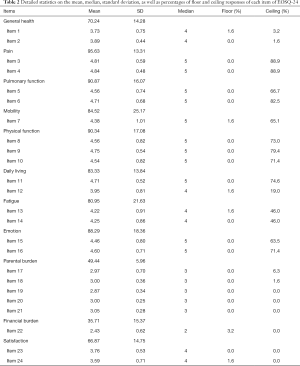
There were no missing values thanks to the dedicated research assistant who was responsible to ascertain completion of the questionnaire. The mean, standard deviation, median, as well as the percentages for floor and ceiling effects were summarized in Table 2. The mean item scores ranged between 2.43 (Item 22) and 4.84 (Item 4) and the mean domain scores ranged between 35.71 (Financial burden) and 95.63 (Pain). Four of the 24 items had evenly distributed responses over the 5 possible answers. Eighteen items were left-skewed towards normal physical and psychosocial well-being, among which 7 (Items 1, 2, 12, 13, 14, 23, 24) had a median value of 4 and 11 items (Items 3, 4, 5, 6, 7, 8, 9, 10, 11, 15, 16) had a median value of 5. Item 22 (financial burden) was the only right-skewed item in our study, with a median value of 2. Floor effect was observed in 0% to 3.2% of all respondents and ceiling effect was observed in 0% to 88.9% of them. Ceiling effect was most significant with the pain-related, pulmonary function-related, and physical function-related items, since the majority of our study subjects were children who had undergone surgical correction. With the exception of those items with high ceiling effects, the inter-item correlations were less than 0.8, indicating good item internal consistency.
Full table
Reliability
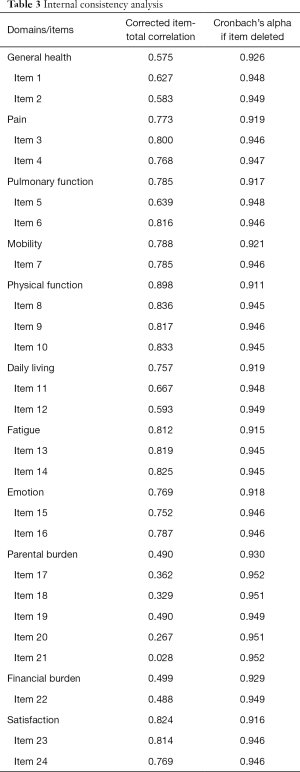
Cronbach’s α coefficient for internal consistency reliability was 0.950 for the 24 items and 0.927 for the 11 domains, both indicating very good reliability. “Cronbach’s α if item deleted” for each item ranged from 0.946 to 0.952; “Cronbach’s α if item deleted” for each domain ranged from 0.911 to 0.930 (Table 3). Corrected item-total correlation coefficients were higher than 0.3 for all items, with the exception of Item 20 (0.267) and Item 21 (0.028); in regards to the 11 domains, the corrected item-total correlation coefficients were all higher than 0.3, ranging from 0.490 to 0.898.
Full table
Discriminative validity
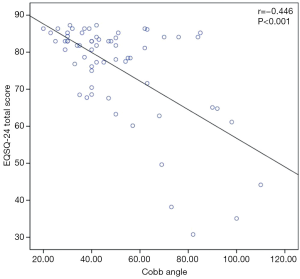
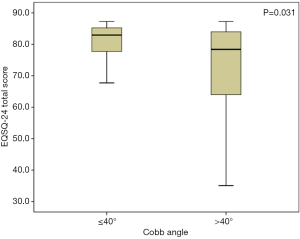
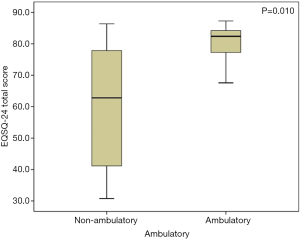
The average total questionnaire score was greater for patients with Cobb angles ≤40° (28 patients) than for those (35 patients) with Cobb angles >40° [83.0 (77.7–85.2) and 78.2 (63.1–83.9), respectively; P=0.031] (Figure 1), indicating an overall significantly better patient-reported HRQoL, in accordance with results from previous studies (9). A Cobb angle of 40 degrees is generally considered a reference point in describing the severity of the scoliotic deformity and was therefore used here as the cutoff value. Specifically, Cobb angle was found significantly affecting Patient Quality of Life items (Items 1–16, P=0.014), but not for the Family burden (Items 17–22, P=0.406) or the Satisfaction (Items 23,24, P=0.232). Furthermore, we found a strong negative correlation between the Cobb angle and the total questionnaire score (rho =−0.446, P<0.001) (Figure 2), suggesting that nearly half of the variation seen in the total questionnaire score could be attributed to curve severity in a linear correlation model. However, whether or not the difference in EOSQ-24 score between patients Cobb angles ≤40° and patients with Cobb angles >40° was clinically meaningful remained to be examined in future studies. Additionally, there was an even more significant difference regarding total questionnaire scores between patients who were ambulatory (52 patients) and those (11 patients) who were non-ambulatory [82.4 (77.3–84.3) and 59.4 (47.1–72.5), respectively; P=0.010] (Figure 3), and this impact was significant for all three domains of Patient Quality of Life (Items 1–16, P=0.011), Family burden (Items 17–22, P=0.018), and Satisfaction (Items 23,24, P=0.001). Different treatment modalities, different diagnoses, age at presentation, and age at surgery were also examined but had no significant impact on the total EOSQ score.
DiscussionOther Section
EOS describes the complex, and often severe, deformity of the spine and thorax affecting children younger than 10 years old as a result of a variety of congenital or medical conditions. Young children with EOS are at risk for impaired lung and heart function because of the high risk of progressive spinal deformity and thoracic constraints during the crucial early period of maximal cardiopulmonary growth and development time of the body. HRQoL in children with EOS has been shown to be significantly impaired, especially in regards to their physical function and caregiver burden. Standard adult HRQoL measures do not exhibit sufficient discriminant validity or criterion validity when utilized to assess the pediatric population (4). Several instruments developed specifically for pediatric conditions have been validated in this patient population (3,4). EOSQ-24 is a disease-specific assessment tool that captures the HRQoL in children with EOS (6,18) to standardize outcome evaluation and facilitate comparison of the efficacy of various controversial and evolving treatment modalities.
In this study, we translated and adapted the original EOSQ-24 into simplified Chinese following international guidelines, and the new instrument demonstrated satisfactory internal consistency reliability and construct validity, similar to findings from previous studies on the Turkish, Spanish, and traditional Chinese versions (8-10). Financial burden was the only item with a right-skewed response distribution (median value =2), indicating the significant difficulties these families withstood. No floor effect was found in any of the questionnaire items but high ceiling effect was observed in those related to pain and physical function. Similarly high ceiling effect was observed in previous studies validating other language versions of EOSQ-24 (8-10), as a result of the relatively high proportion of EOS children who were in the recovery stage (post-operative) of the disease at the time of assessment. Scores of the simplified Chinese version of EOSQ-24 were statistically different between ambulatory and non-ambulatory patients as well as between those with different levels of curve severity. These preliminary results were suggestive of satisfactory discriminative validity of the translated scale but further studies warranted for a more definitive conclusion in this regard. Strong negative correlations were found between the scores of all three domains (Patient Quality of Life, Family burden, and Satisfaction) and patient ambulatory status, and between the total questionnaire score and curve severity as determined by Cobb angle. However, the majority (81%) of our subjects had congenital scoliosis; therefore, the study was underpowered to detect HRQoL differences between different etiologies. Additionally, the high extent of economic difficulties related to EOS as identified in our study might not be representative of the real financial burden of all families with EOS children in mainland China, as all subjects in this study were recruited from one province. Further studies are also warranted to evaluate the test-retest reliability and its responsiveness to different treatment modalities.
The sample size of this study was limited by the low incidence of EOS, yet comparable to that in some of the previous studies in other patient populations [e.g., sample size was 44 in the Spanish version (9), 61 in the Turkish version (8), 55 in the Norwegian version (19), and 58 in the Arabic version (20)]. Internal consistency was assessed in all previous studies, yet only the Dutch (21), the Turkish (8), and the traditional Chinese version (10) studies evaluated construct validity. The Spanish (9), the Norwegian (19), and the Arabic (20), and the German version (22) studies evaluated discriminative validity in addition to floor and ceiling effects of the translated instrument, similar to what was performed in the current study. Comparison of EOSQ-24 scores between the EOS and healthy control groups was not performed in any of the above translation and cultural adaptation studies, which all demonstrated the reliability and validity of EOSQ-24 in their respective languages with minimal cultural difference, suggesting high generalizability of this instrument.
ConclusionsOther Section
The simplified Chinese version of EOSQ-24 is a reliable and valid tool for HRQoL assessment among caregivers (parents) of children with EOS in mainland China. It demonstrated satisfactory psychometric properties similar to those observed with the original instrument, and could therefore potentially be incorporated into routine clinical and adopted as a standard assessment tool for research purposes. However, future studies are warranted to evaluate the test-retest reliability of the simplified Chinese version of EOSQ-24, its responsiveness to different treatment modalities, and to further validate its discriminative validity.
AcknowledgmentsOther Section
Funding: This is study was supported by a grant from Capital’s Fund for Health Development and Research (Grant No. 2018-4-4097) and a grant from Young Scientists Fund of the National Natural Science Foundation of China (Grant No. 2019NSFC81901822). The organizations that funded this study had no role in the study design, data collection and analysis or the decision to prepare or publish the manuscript.
FootnoteOther Section
Reporting Checklist: The authors have completed the reporting checklist. Available at http://dx.doi.org/10.21037/tp-19-177
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tp-19-177
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/tp-19-177). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Peking University Third Hospital (No. M2015115) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Pehrsson K, Larsson S, Oden A, et al. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine (Phila Pa 1976) 1992;17:1091-6. [Crossref] [PubMed]
- Vitale MG, Matsumoto H, Roye DP Jr, et al. Health-related quality of life in children with thoracic insufficiency syndrome. J Pediatr Orthop 2008;28:239-43. [Crossref] [PubMed]
- Vitale MG, Levy DE, Moskowitz AJ, et al. Capturing quality of life in pediatric orthopaedics: two recent measures compared. J Pediatr Orthop 2001;21:629-35. [Crossref] [PubMed]
- Vitale MG, Roye EA, Choe JC, et al. Assessment of health status in patients with cerebral palsy: what is the role of quality-of-life measures? J Pediatr Orthop 2005;25:792-7. [Crossref] [PubMed]
- Matsumoto H, Williams B, Park HY, et al. The Final 24-Item Early Onset Scoliosis Questionnaires (EOSQ-24): Validity, Reliability and Responsiveness. J Pediatr Orthop 2018;38:144-51. [Crossref] [PubMed]
- Corona J, Matsumoto H, Roye DP, et al. Measuring quality of life in children with early onset scoliosis: development and initial validation of the early onset scoliosis questionnaire. J Pediatr Orthop 2011;31:180-5. [Crossref] [PubMed]
- Beaton DE, Bombardier C, Guillemin F, et al. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25:3186-91. [Crossref] [PubMed]
- Demirkiran HG, Kinikli GI, Olgun ZD, et al. Reliability and Validity of the Adapted Turkish Version of the Early-onset Scoliosis-24-Item Questionnaire (EOSQ-24). J Pediatr Orthop 2015;35:804-9. [PubMed]
- Del Mar Pozo-Balado M, Matsumoto H, Vitale MG, et al. Reliability and Validity of the Adapted Spanish Version of the Early Onset Scoliosis-24 Questionnaire. Spine (Phila Pa 1976) 2016;41:E625-31. [Crossref] [PubMed]
- Cheung JP, Cheung PW, Wong CK, et al. Psychometric Validation of the Traditional Chinese Version of the Early Onset Scoliosis-24 Item Questionnaire (EOSQ-24). Spine (Phila Pa 1976) 2016;41:E1460-9. [Crossref] [PubMed]
- Cheung KM, Senkoylu A, Alanay A, et al. Reliability and concurrent validity of the adapted Chinese version of Scoliosis Research Society-22 (SRS-22) questionnaire. Spine (Phila Pa 1976) 2007;32:1141-5. [Crossref] [PubMed]
- Guo J, Lau AH, Chau J, et al. A validation study on the traditional Chinese version of Spinal Appearance Questionnaire for adolescent idiopathic scoliosis. Eur Spine J 2016;25:3186-93. [Crossref] [PubMed]
- Li M, Wang CF, Gu SX, et al. Adapted simplified Chinese (mainland) version of Scoliosis Research Society-22 questionnaire. Spine (Phila Pa 1976) 2009;34:1321-4. [Crossref] [PubMed]
- Wei X, Zhu X, Bai Y, et al. Development of the Simplified Chinese Version of the Spinal Appearance Questionnaire: cross-cultural adaptation and psychometric properties evaluation. Spine (Phila Pa 1976) 2012;37:1497-504. [Crossref] [PubMed]
- Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 1993;46:1417-32. [Crossref] [PubMed]
- Ware JE Jr. Conceptualizing and measuring generic health outcomes. Cancer 1991;67:774-9. [Crossref] [PubMed]
- Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60:34-42. [Crossref] [PubMed]
- Vitale MG, Corona J, Matsumoto H, et al. Development and initial validation of a disease specific outcome measure for early onset scoliosis. Stud Health Technol Inform 2010;158:172-6. [PubMed]
- Molland RS, Diep LM, Brox JI, et al. Reliability and Construct Validity of the Adapted Norwegian Version of the Early-Onset Scoliosis 24-item Questionnaire. J Am Acad Orthop Surg Glob Res Rev 2018;2:e066. [PubMed]
- Hanbali Y, Perry T, Hanif A, et al. Reliability and validity of the Arabic version of the Early Onset Scoliosis 24 Items Questionnaire (EOSQ-24). SICOT J 2019;5:7. [Crossref] [PubMed]
- Wijdicks SPJ, Dompeling SD, de Reuver S, et al. Reliability and Validity of the Adapted Dutch Version of the Early-Onset Scoliosis-24-Item Questionnaire (EOSQ-24). Spine (Phila Pa 1976) 2019;44:E965-73. [Crossref] [PubMed]
- Mladenov K, Braunschweig L, Behrend J, et al. Validation of the German version of the 24-item Early-Onset Scoliosis Questionnaire. J Neurosurg Pediatr 2019. [Crossref] [PubMed]