
Fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia: a narrative review of the history, current practice, and future directions
Introduction
Congenital diaphragmatic hernia (CDH) is a defect in the fetal diaphragm that allows organs within the abdomen to migrate into the thorax which leads to a combination of pulmonary hypoplasia and pulmonary hypertension (1,2). The condition arises in the embryonic period, so that lung development can already be impaired from early in pregnancy (2). It is diagnosed prenatally 68% of the time and in many series the outcome for these fetuses is associated with lower survival and less favorable long-term outcomes than those diagnosed postnatally, especially when diagnosed at an earlier gestational age (3-5). This coincides with larger defect sizes being diagnosed prenatally which correlate to higher morbidity and mortality (3). Despite advances in neonatal care and resuscitation, survival rates as described by the CDH study group (CDHSG) are 71% when diagnosed prenatally and 83% when diagnosed postnatally (3). The CDHSG was developed in 1995 as a multi-center, international data collection initiative for live-born infants with CDH and used to advance knowledge and develop evidence-based solutions for clinical questions (6,7).
Prenatal ultrasound and fetal magnetic resonance imaging (MRI) are used to estimate lung volumes in the antenatal period and provide anticipatory counseling. Ultrasound utilizes the lung to head ratio (LHR), observed-to-expected lung to head ratio (o/e LHR), the position of the liver as “up” in the thorax versus “down” in the abdomen, and more recently stomach position (8-10). The LHR is calculated by measuring the area of the contralateral lung at the level of the four-chamber view of the heart divided by the head circumference (11). Since the gestational age affects the growth of the lung and head differently, LHR increases with gestational age. Therefore, the o/e LHR was developed based on the expected LHR for a given gestational age which has the advantage of remaining more constant throughout gestation (8). Currently, within the framework of a clinical trial, severe pulmonary hypoplasia is defined as corresponding with an o/e LHR <25% in isolated left-sided cases, correlating with a <20% survival rate (9). Fetal MRI is a useful adjunct in prenatal workup to measures fetal lung volumes with a variety of equations used to calculate “normal” lung volumes for gestational age (12). These are most commonly expressed as observed-to-expected total fetal lung volume (o/e TFLV) or percent predicted lung volume (PPLV) (13,14). An o/e TFLV with a value <25% and a PPLV <15–25% are predictive of higher mortality (14-16).
There has been much interest and investigation into fetal treatment for CDH given its associated postnatal morbidity and mortality. The objective of this review is to provide the reader with information on the history of fetal intervention for CDH, current fetal endoscopic tracheal occlusion (FETO) practices with reported outcomes, and future directions. For the review of outcomes, we have included studies that were completed in humans with comparison groups and were available in English or with English-translation. We present the following article in accordance with the narrative review reporting checklist (available at http://dx.doi.org/10.21037/tp-20-130).
Historical perspective
Due to the severity of the disease and high mortality, Dr. Michael Harrison and his team at the University of San Francisco (UCSF) began the study into fetal treatment of diaphragmatic hernia in the 1990s (17,18). Fetal anatomical repair through maternal laparotomy and hysterotomy, although operatively successful in liver-down CDH, led to increased rates of premature birth and no difference in survival when compared to postnatal repair (19). Open fetal surgery for liver-up CDH, the more severe cohort, was abandoned as it had a high risk of fetal death during the operation due to kinking of the umbilical vein when the liver was returned to the abdomen (18). Due to these outcomes, this strategy was stopped as other strategies for prenatal intervention were explored. It was known that fetuses with congenital high airway obstruction syndrome (CHAOS) had hyperplastic lungs at birth and fetal rabbit studies from 1965 showed that tracheal ligation led to increased lung size (20). Later studies confirmed that tracheal occlusion could lead to improved lung volume as well as functional improvement in the lung in the fetal CDH lamb model (21-25). Animal models also showed a benefit to removing the balloon prior to delivery (plug-unplug sequence) as this normalizes the number of type II pneumocytes in the alveoli which are decreased if tracheal ligation remains until birth (26,27). Therefore, techniques for tracheal occlusion in humans were subsequently developed, including those that were endoscopic and amenable to easy reversal of occlusion, since temporary occlusion is associated with improved lung maturation (26,28,29).
Clinically, sustained tracheal occlusion was originally performed via open hysterotomy. In the first series of eight patients, an external tracheal clip (n=6) or internal foam plug (n=2) was used for occlusion. In this series from UCSF, four fetuses showed dramatic in-utero lung growth despite only a single survivor (30). Interestingly, tracheal damage was most significant with the internal plug as they required tracheotomy for removal while the external clips produced minimal tracheal damage (30). These cases all required an ex utero intrapartum treatment (EXIT) procedure in order to remove the plug or clip on placental bypass prior to full Cesarean delivery (31). Tracheal occlusion was also done via open hysterotomy, neck dissection, and external clip application in 15 patients at the Children’s Hospital of Philadelphia (CHOP) with a survival rate of 33% (5/15) (32). Although this clinical experience demonstrated that tracheal occlusion induces lung growth, open hysterotomy techniques were plagued with premature birth and therefore fetoscopic tracheal occlusion was investigated (30,33,34). Simultaneously, endoluminal tracheal occlusion was being studied in animal models with a variety of different devices utilized including foam plugs, magnetic valves, a self-expanding umbrella, and, finally, vascular occlusion balloons, all with the purpose to make reversal easy (29,35,36).
Human trials shifted to endoscopic tracheal occlusion after maternal laparotomy, or the Fetendo Clip, as it was originally named (34), and had impressive results in the first 8 patients at the UCSF. This group had a 75% survival rate compared with 15% in the open tracheal occlusion group (n=13) and 38% in the postnatal treatment group (33). Given these promising findings, a randomized trial was initiated and recruited pregnant mothers carrying fetuses with liver-up CDH who had isolated CDH and were on the moderate to severe spectrum (LHR <1.4 between 22 and 27 weeks) (37). For this trial, the technique evolved from external metallic clips to the detachable endoluminal balloon following maternal laparotomy using a single port of 4.5mm diameter into the exposed uterus (38). Despite initial excitement, the trial was stopped by the data safety monitoring board after 24 patients due to a higher than expected survival at 90 days of age in the expectantly managed group (8/11, 73%) and no better in the tracheal occlusion group (10/13, 77%) (37). The improved survival in the untreated group was thought to be due to improved perinatal care during the study period and inclusion of fetuses with moderate hypoplasia (LHR 1.0–1.4) (37).
The clinical percutaneous approach to tracheal occlusion was first reported as a single case report by Quintero et al. but the infant did not survive due to device failure (39). In Europe, the FETO Task Force developed a percutaneous technique using a 3.3 mm diameter uterine access, initially under general anesthesia and evolving to loco-regional and later local anesthesia (40,41). Their first-in-woman trial used this percutaneous approach and recruited patients with LHR <1.0 between 25 and 29 weeks gestation (42,43). Selection criteria were later modified to correct for gestational age in outcome prediction, hence using the o/e LHR rather than the LHR, which is gestational age dependent (9). In 2009 this consortium reported survival rates increasing from 24.1% (expected) to 49.1% (FETO) in left-sided CDH and from 0% (expected) to 35.3% (FETO) in right-sided CDH (42). A later study confirmed better outcomes in cases of right-sided CDH (44). There is also some evidence that FETO reduces early neonatal morbidity (45,46). Results from this trial have been criticized, however, as the comparison group was historical rather than contemporaneous and the expected percentages for survival were therefore low. In Brazil, a randomized control trail of FETO versus control for LHR <1.0 showed significant improvement in survival with FETO (10/19, 52.6%) versus controls (1/19, 5.3%) (47). This trial was even more criticized as it merged left and right-sided cases, the balloon was left until delivery, there was an unexplainable difference in the numbers in the treatment group, and the extremely low survival rate in the expectantly managed group (48).
Due to these varying results, the fetal community has agreed that currently there is insufficient evidence to recommend fetal intervention as the standard of care (49,50). The Tracheal Occlusion to Accelerate Lung Growth (TOTAL) trial (www.totaltrial.eu; ClinicalTrials.gov, NCT01240057and NCT00763737) is a randomized control trial of FETO vs. expectant postnatal management followed by standardized postnatal care initiated in Europe, and meanwhile extended to Australia, the United States, and Japan (51). At this time, the severe arm of the trial continues to enroll patients while the moderate arm is completed with enrollment and awaiting final analysis of endpoints.
Procedure description
The current procedure utilizes a fetoscopic approach with a detachable balloon that is placed midgestation and removed around 34 weeks (see Figure 1) (41,52,53). Inclusion criteria vary slightly at different centers but typically include singleton pregnancy and isolated CDH with normal chromosomal and no other associated anomalies. The GOLDBAL2 (Balt Extrusion, Montmorency, France) is a latex balloon with a one-way valve that was originally designed for endovascular use. It is used off-label and it measures 7.0×20 mm2 when inflated (see Figures 2,3) (53,54). The procedure can be performed under local anesthesia with or without maternal sedation and fetal anesthesia (e.g., fentanyl, atropine, and vecuronium, or equivalent neuromuscular blocking agent) to ensure minimal fetal movement. Balloon placement is typically done between weeks 27–29 weeks in severe cases and later (30–32 weeks) for moderate cases (53). A 3.3 mm cannula is placed percutaneously (i.e., through the maternal skin, abdominal wall, and uterus) and directed towards the fetal mouth, taking care to avoid the placenta to gain fetoscopic access. The deflated balloon on the delivery microcatheter (BALTACCI-BDPE100) is preloaded into the fetal tracheoscopic sheath (11540KE; Karl Storz) (see Figure 3A). This sheath was designed specifically for FETO and has 3 side ports to allow all necessary equipment to be available for placement and retrieval: camera (11540AA; Karl Storz), balloon with microcatheter, adjustable puncture needle (11506P; Karl Storz), and forceps (11510C; Karl Storz) (see Figure 4). Adequate alignment to the fetal mouth is required and guided with ultrasound (see Figure 1). The fetoscope is placed into the fetal mouth and the operator carefully visualizes the tongue, uvula, epiglottis, and vocal cords (see Figure 5). Once past the vocal cords, the balloon is positioned above the carina and 0.6 mL of saline is used for inflation. The inflated balloon is detached from the microcatheter and verification of correct position is completed with ultrasound (53,55).
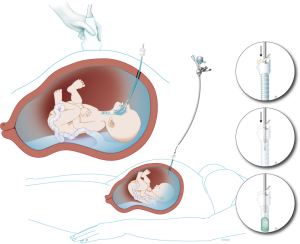


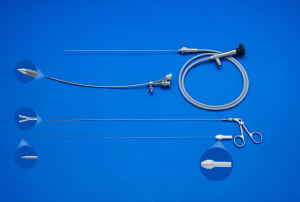
Ideally, the balloon remains in place until 34 weeks with weekly maternal follow up to monitor for potential deflation of the balloon and complications, most commonly polyhydramnios, chorioamniotic membrane separation, preterm rupture of membranes, chorioamnionitis, or other signs of preterm labor (53). Balloon removal is performed via fetoscopic retrieval, percutaneous puncture using ultrasound-guidance, or tracheoscopic removal on placental circulation during standard cesarean section or, at last resort, in the immediate neonatal period (56). The method of balloon removal depends upon the expertise at each center, the accessibility of the balloon for ultrasound guided puncture, and the stability of the mother and fetus at the time of removal. The fetus is anesthetized and, for fetoscopic removal, a forceps and an adjustable puncture needle is utilized (Figure 3B,C). Once the balloon is removed, the pregnancy can be managed expectantly and women can deliver vaginally.
Outcomes
Despite our current inability to recommend FETO as standard of care as described above, much has been learned from the patients that have undergone this fetal intervention. Lung size increases in a majority of patients and echogenic changes are visualized within 48 hours of balloon placement (40,57). The increase in lung size is dependent on initial o/e LHR and the timing of the occlusion and balloon removal (58,59). The tracheal balloon has local side effects, both on the epithelium and causes widening of the trachea, which decreases with increasing age (52,60). In some patients, a temporary barking cough on effort has been described (61). The gestational age at insertion is also related to the degree of tracheomegaly, and early insertions may cause more clinical problems (62,63).
The most commonly reported neonatal outcomes are survival and gestational age (GA) at delivery with comparative studies consistently reporting earlier GA at delivery for FETO and mixed results around survival (see Table 1) (37,40,42,47,57,64-71). A few studies report rates of extracorporeal membrane oxygenation (ECMO) utilization and severe pulmonary hypertension and that neonates who received FETO have lower rates of both (47,65,67,68,71). Other neonatal outcomes (including need for respiratory support and length of stay) are even more scarce but included in Table 1 when available.
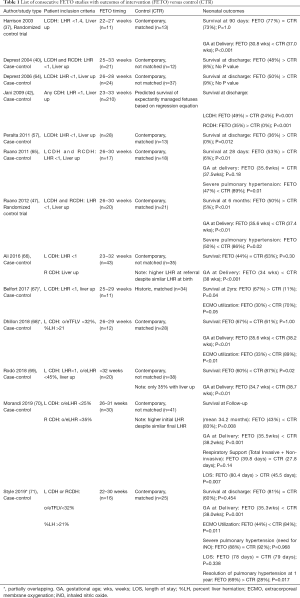
Full table
A couple of recent systematic reviews have attempted to answer questions regarding outcomes (72-75). A 2016 systematic review (total of 5 studies; n=110 FETO and n=101 control) concluded that FETO favored increased survival [OR 13.32 (5.40–32.87)] and associated preterm birth with a mean gestational age of 35.6 weeks (72). A 2017 systematic review including expanded studies (18 studies; n=854 FETO cases) and concluded that preterm birth is a significant complication of FETO with 72% of patients delivering <37 weeks and nearly 18% delivering <32 weeks (73). Premature rupture of membranes has been seen in nearly 47% of cases while chorioamnionitis and placental abruption was recorded in 2.6% and 1.2% of cases, respectively (73). The authors also concluded that FETO increased neonatal survival at 30 days [RR 5.8 (1.5–22.9) days] and 6 months [RR 10.5 (1.5–74.7) months] but led to a higher rate of premature rupture of membranes [RR 1.7 (0.8–2.4)] and decreased gestational age at delivery by nearly 2 weeks (73). In a 2019 systematic review on maternal complications of fetoscopic surgery (122 studies; n=9,403 patients), the rate of complications was 6.15% (4.93–7.49%), including abruption as the most common severe complication (1.29%) with most being minor complications (4.33%) (74). Despite these reviews, critics question the validity given that data is coming from the same groups and were not properly controlled.
The TOTAL trial results are expected in the near future and many are anxiously awaiting the results. However, there have been concerns regarding the slow enrollment and leakage of patient’s out of the trial (76). Despite these limitations, this will be the largest randomized control trial of fetal therapy to date and includes contemporary, matched controls. Together with standardization of CDH management and improvement of postnatal resuscitation, fetal intervention remains an exciting adjunct of care for this complex disease (40,52,57-63).
Future directions
Currently FETO is being offered for cases of isolated CDH, i.e., without associated fetal anomalies, due to the experimental nature of the procedure. Some have argued for expansion to include other anomalies as 25% of fetuses have other organ anomalies, most commonly cardiac defects or congenital lung lesions (77). A single institution demonstrated favorable outcomes when including selective non-isolated cases of severe CDH utilizing a compassionate use exemption, including a single case of Tetralogy of Fallot identified post-balloon and congenital lung lesion identified prior to procedure (77). If the TOTAL trial demonstrates efficacy for FETO in isolated CDH, there may be rationale for expanding inclusion criteria to patients with certain additional anomalies.
A new “Smart-TO” balloon is in development that would avoid the need for a second procedure for puncture and retrieval of the balloon. This balloon utilizes a magnetic valve that can be activated in the fringe magnetic field of an MRI machine (78). The tracheal effects are similar to those of the currently used BALT balloon (79).
Although data suggests that tracheal occlusion stimulates lung growth, there is no convincing evidence that there is also concomitant vascular growth and remodeling, that might ameliorate pulmonary hypertension. Pulmonary hypertension is the second most important cause of death for these patients. Transplacental sildenafil, a phosphodiesterase-5 inhibitor, has been shown effective in several animal models (80,81). It is currently being tested in a phase I trial and may work synergistically with tracheal occlusion (82,83). Obviously, a pharmacologic approach would make prenatal therapy much more widely accessible (55).
Conclusions
There has been much work done within the past 40 years to study fetal intervention for CDH. This review focuses on what is known to date with reported outcomes of human trials and includes a look into future directions.
Acknowledgments
The authors would like to thank the principal investigators and centers who have been part of the TOTAL trial including: J. Deprest, UZ Leuven, Leuven, Belgium; E. Gratacos, Hospital Clinic, Barcelona, Spain; K. Nicolaides, King’s Hospital, London; A. Benachi, Béclère, AP Hôpitaux Paris, France; Y. Ville, Necker, AP Hôpitaux Paris, France; C. Berg, Uniklinik Bonn, Germany; G. Gardener, Mater Hospital, Brisbane, Australia; G. Ryan, Mount Sinai Hospital, Toronto, Canada; N. Persico, Maggiori Hospital, Milan, Italy; P. Bagolan, Bambinu Gesù, Rome, Italy; M. Belfort, Baylor College, Houston, Texas, USA; H. Sago, Tokyo, Japan; M Wielgos, Warsaw, Poland; H. Hedrick, CHOP, Philadelphia, PA, USA; A. Johnson, UTSHC, Houston, Texas, USA; F.Y. Lim, CCHMC, Cincinnati, OH, USA.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Eric B. Jelin and George B. Mychaliska) for the series “Fetal Surgery” published in Translational Pediatrics. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/tp-20-130
Peer Review File: Available at http://dx.doi.org/10.21037/tp-20-130
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-130). The series “Fetal Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bianchi DW CT, D'Alton ME, Malone FD, eds. Fetology. 2nd Edition ed. New York, NY: McGraw-Hill, 2010.
- Campanale RP, Rowland RH. Hypoplasia of the lung associated with congenital diaphragmatic hernia. Ann Surg 1955;142:176-89. [Crossref] [PubMed]
- Burgos CM, Frenckner B, Luco M, et al. Prenatally versus postnatally diagnosed congenital diaphragmatic hernia - Side, stage, and outcome. J Pediatr Surg 2019;54:651-5. [Crossref] [PubMed]
- Gallot D, Boda C, Ughetto S, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol 2007;29:276-83. [Crossref] [PubMed]
- Bouchghoul H, Senat MV, Storme L, et al. Congenital diaphragmatic hernia: does gestational age at diagnosis matter when evaluating morbidity and mortality? Am J Obstet Gynecol 2015;213:535.e1-7. [Crossref] [PubMed]
- Doyle NM, Lally KP. The CDH Study Group and advances in the clinical care of the patient with congenital diaphragmatic hernia. Semin Perinatol 2004;28:174-84. [Crossref] [PubMed]
- Harting MT, Lally KP. The Congenital Diaphragmatic Hernia Study Group registry update. Semin Fetal Neonatal Med 2014;19:370-5. [Crossref] [PubMed]
- Jani J, Nicolaides KH, Keller RL, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol 2007;30:67-71. [Crossref] [PubMed]
- Deprest JA, Flemmer AW, Gratacos E, et al. Antenatal prediction of lung volume and in-utero treatment by fetal endoscopic tracheal occlusion in severe isolated congenital diaphragmatic hernia. Semin Fetal Neonatal Med 2009;14:8-13. [Crossref] [PubMed]
- Cordier AG, Cannie MM, Guilbaud L, et al. Stomach position versus liver-to-thoracic volume ratio in left-sided congenital diaphragmatic hernia. J Matern Fetal Neonatal Med 2015;28:190-5. [Crossref] [PubMed]
- Metkus AP, Filly RA, Stringer MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg 1996;31:148-51; discussion 151-2. [Crossref] [PubMed]
- Deshmukh S, Rubesova E, Barth R. MR assessment of normal fetal lung volumes: a literature review. AJR Am J Roentgenol 2010;194:W212-7 [Crossref] [PubMed]
- Kim AG, Mon RA, Karmakar M, et al. Calculating Observed-to-Expected Total Fetal Lung Volume in CDH Fetuses in Twin Gestation: Is There a Better Way? Fetal Diagn Ther 2020;47:545-53. [Crossref] [PubMed]
- Barnewolt CE, Kunisaki SM, Fauza DO, et al. Percent predicted lung volumes as measured on fetal magnetic resonance imaging: a useful biometric parameter for risk stratification in congenital diaphragmatic hernia. J Pediatr Surg 2007;42:193-7. [Crossref] [PubMed]
- Oluyomi-Obi T, Kuret V, Puligandla P, et al. Antenatal predictors of outcome in prenatally diagnosed congenital diaphragmatic hernia (CDH). J Pediatr Surg 2017;52:881-8. [Crossref] [PubMed]
- Victoria T, Bebbington MW, Danzer E, et al. Use of magnetic resonance imaging in prenatal prognosis of the fetus with isolated left congenital diaphragmatic hernia. Prenat Diagn 2012;32:715-23. [Crossref] [PubMed]
- Harrison MR, Adzick NS, Longaker MT, et al. Successful repair in utero of a fetal diaphragmatic hernia after removal of herniated viscera from the left thorax. N Engl J Med 1990;322:1582-4. [Crossref] [PubMed]
- Harrison MR, Langer JC, Adzick NS, et al. Correction of congenital diaphragmatic hernia in utero, V. Initial clinical experience. J Pediatr Surg 1990;25:47-55; discussion 56-7. [Crossref] [PubMed]
- Harrison MR, Adzick NS, Bullard KM, et al. Correction of congenital diaphragmatic hernia in utero VII: a prospective trial. J Pediatr Surg 1997;32:1637-42. [Crossref] [PubMed]
- Carmel JA, Friedman F, Adams FH. Fetal tracheal ligation and lung development. Am J Dis Child 1965;109:452-6. [PubMed]
- DiFiore JW, Fauza DO, Slavin R, et al. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg 1994;29:248-56; discussion 256-7. [Crossref] [PubMed]
- Hedrick MH, Estes JM, Sullivan KM, et al. Plug the lung until it grows (PLUG): a new method to treat congenital diaphragmatic hernia in utero. J Pediatr Surg 1994;29:612-7. [Crossref] [PubMed]
- Beierle EA, Langham MR Jr, Cassin S. In utero lung growth of fetal sheep with diaphragmatic hernia and tracheal stenosis. J Pediatr Surg 1996;31:141-6; discussion 146-7. [Crossref] [PubMed]
- Skarsgard ED, Meuli M, VanderWall KJ, et al. Fetal endoscopic tracheal occlusion ('Fetendo-PLUG') for congenital diaphragmatic hernia. J Pediatr Surg 1996;31:1335-8. [Crossref] [PubMed]
- Deprest JA, Evrard VA, Van Ballaer PP, et al. Tracheoscopic endoluminal plugging using an inflatable device in the fetal lamb model. Eur J Obstet Gynecol Reprod Biol 1998;81:165-9. [Crossref] [PubMed]
- Flageole H, Evrard VA, Piedboeuf B, et al. The plug-unplug sequence: an important step to achieve type II pneumocyte maturation in the fetal lamb model. J Pediatr Surg 1998;33:299-303. [Crossref] [PubMed]
- Nelson SM, Hajivassiliou CA, Haddock G, et al. Rescue of the hypoplastic lung by prenatal cyclical strain. Am J Respir Crit Care Med 2005;171:1395-402. [Crossref] [PubMed]
- Evrard VA, Flageole H, Deprest JA, et al. Intrauterine tracheal obstruction, a new treatment for congenital diaphragmatic hernia, decreases amniotic fluid sodium and chloride concentrations in the fetal lamb. Ann Surg 1997;226:753-8. [Crossref] [PubMed]
- Flageole H, Evrard VA, Vandenberghe K, et al. Tracheoscopic endotracheal occlusion in the ovine model: technique and pulmonary effects. J Pediatr Surg 1997;32:1328-31. [Crossref] [PubMed]
- Harrison MR, Adzick NS, Flake AW, et al. Correction of congenital diaphragmatic hernia in utero VIII: Response of the hypoplastic lung to tracheal occlusion. J Pediatr Surg 1996;31:1339-48. [Crossref] [PubMed]
- Hirose S, Harrison MR. The ex utero intrapartum treatment (EXIT) procedure. Semin Neonatol 2003;8:207-14. [Crossref] [PubMed]
- Flake AW, Crombleholme TM, Johnson MP, et al. Treatment of severe congenital diaphragmatic hernia by fetal tracheal occlusion: clinical experience with fifteen cases. Am J Obstet Gynecol 2000;183:1059-66. [Crossref] [PubMed]
- Harrison MR, Mychaliska GB, Albanese CT, et al. Correction of congenital diaphragmatic hernia in utero IX: fetuses with poor prognosis (liver herniation and low lung-to-head ratio) can be saved by fetoscopic temporary tracheal occlusion. J Pediatr Surg 1998;33:1017-22; discussion 1022-3. [Crossref] [PubMed]
- VanderWall KJ, Bruch SW, Meuli M, et al. Fetal endoscopic ('Fetendo') tracheal clip. J Pediatr Surg 1996;31:1101-3; discussion 1103-4. [Crossref] [PubMed]
- Bealer JF, Skarsgard ED, Hedrick MH, et al. The 'PLUG' odyssey: adventures in experimental fetal tracheal occlusion. J Pediatr Surg 1995;30:361-4; discussion 364-5. [Crossref] [PubMed]
- Luks FI, Gilchrist BF, Jackson BT, et al. Endoscopic tracheal obstruction with an expanding device in a fetal lamb model: preliminary considerations. Fetal Diagn Ther 1996;11:67-71. [Crossref] [PubMed]
- Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med 2003;349:1916-24. [Crossref] [PubMed]
- Harrison MR, Albanese CT, Hawgood SB, et al. Fetoscopic temporary tracheal occlusion by means of detachable balloon for congenital diaphragmatic hernia. Am J Obstet Gynecol 2001;185:730-3. [Crossref] [PubMed]
- Quintero R, Bornick PW, Allen MH, Johnson PK. Minimally invasive intraluminal tracheal occlusion in a human fetus with left sided congenital diaphragmatic hernia at 27 weeks via direct fetal laryngoscopy. Prenatal and Neonatal Medicine 2000;5:134-40.
- Deprest J, Gratacos E, Nicolaides KH. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol 2004;24:121-6. [Crossref] [PubMed]
- Deprest J, Jani J, Gratacos E, et al. Fetal intervention for congenital diaphragmatic hernia: the European experience. Semin Perinatol 2005;29:94-103. [Crossref] [PubMed]
- Jani JC, Nicolaides KH, Gratacos E, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol 2009;34:304-10. [Crossref] [PubMed]
- Jani JC, Nicolaides KH, Gratacos E, et al. Fetal lung-to-head ratio in the prediction of survival in severe left-sided diaphragmatic hernia treated by fetal endoscopic tracheal occlusion (FETO). Am J Obstet Gynecol 2006;195:1646-50. [Crossref] [PubMed]
- DeKoninck P, Gomez O, Sandaite I, et al. Right-sided congenital diaphragmatic hernia in a decade of fetal surgery. BJOG 2015;122:940-6. [Crossref] [PubMed]
- Doné E, Gratacos E, Nicolaides KH, et al. Predictors of neonatal morbidity in fetuses with severe isolated congenital diaphragmatic hernia undergoing fetoscopic tracheal occlusion. Ultrasound Obstet Gynecol 2013;42:77-83. [Crossref] [PubMed]
- Done E, Debeer A, Gucciardo L, et al. Prediction of neonatal respiratory function and pulmonary hypertension in fetuses with isolated congenital diaphragmatic hernia in the fetal endoscopic tracleal occlusion era: a single-center study. Fetal Diagn Ther 2015;37:24-32. [Crossref] [PubMed]
- Ruano R, Yoshisaki CT, da Silva MM, et al. A randomized controlled trial of fetal endoscopic tracheal occlusion versus postnatal management of severe isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2012;39:20-7. [Crossref] [PubMed]
- Jani JC, Nicolaides KH. Fetal surgery for severe congenital diaphragmatic hernia? Ultrasound Obstet Gynecol 2012;39:7-9. [Crossref] [PubMed]
- Deprest JA, Hyett JA, Flake AW, et al. Current controversies in prenatal diagnosis 4: Should fetal surgery be done in all cases of severe diaphragmatic hernia? Prenat Diagn 2009;29:15-9. [Crossref] [PubMed]
- Rodrigues HC, Deprest J. v d Berg PP. When referring physicians and researchers disagree on equipoise: the TOTAL trial experience. Prenat Diagn 2011;31:589-94. [Crossref] [PubMed]
- Deprest J, Brady P, Nicolaides K, et al. Prenatal management of the fetus with isolated congenital diaphragmatic hernia in the era of the TOTAL trial. Semin Fetal Neonatal Med 2014;19:338-48. [Crossref] [PubMed]
- Deprest JA, Evrard VA, Verbeken EK, et al. Tracheal side effects of endoscopic balloon tracheal occlusion in the fetal lamb model. Eur J Obstet Gynecol Reprod Biol 2000;92:119-26. [Crossref] [PubMed]
- Van der Veeken L, Russo FM, De Catte L, et al. Fetoscopic endoluminal tracheal occlusion and reestablishment of fetal airways for congenital diaphragmatic hernia. Gynecol Surg 2018;15:9. [Crossref] [PubMed]
- Niu Y, Li L, Tang J, et al. Embolization of direct carotid cavernous fistulas with the novel double-balloon technique. Interv Neuroradiol 2016;22:201-5. [Crossref] [PubMed]
- Russo FM, De Coppi P, Allegaert K, et al. Current and future antenatal management of isolated congenital diaphragmatic hernia. Semin Fetal Neonatal Med 2017;22:383-90. [Crossref] [PubMed]
- Jimenez JA, Eixarch E, DeKoninck P, et al. Balloon removal after fetoscopic endoluminal tracheal occlusion for congenital diaphragmatic hernia. Am J Obstet Gynecol 2017;217:78.e1-11. [Crossref] [PubMed]
- Peralta CF, Sbragia L, Bennini JR, et al. Tracheal occlusion for fetuses with severe isolated left-sided diaphragmatic hernia: a nonrandomized controlled experimental study. Rev Bras Ginecol Obstet 2011;33:381-7. [PubMed]
- Peralta CF, Jani JC, Van Schoubroeck D, et al. Fetal lung volume after endoscopic tracheal occlusion in the prediction of postnatal outcome. Am J Obstet Gynecol 2008;198:60.e1-5. [Crossref] [PubMed]
- Nawapun K, Eastwood MP, Diaz-Cobos D, et al. In vivo evidence by magnetic resonance volumetry of a gestational age dependent response to tracheal occlusion for congenital diaphragmatic hernia. Prenat Diagn 2015;35:1048-56. [Crossref] [PubMed]
- Breysem L, Debeer A, Claus F, et al. Cross-sectional study of tracheomegaly in children after fetal tracheal occlusion for severe congenital diaphragmatic hernia. Radiology 2010;257:226-32. [Crossref] [PubMed]
- Fayoux P, Hosana G, Devisme L, et al. Neonatal tracheal changes following in utero fetoscopic balloon tracheal occlusion in severe congenital diaphragmatic hernia. J Pediatr Surg 2010;45:687-92. [Crossref] [PubMed]
- Deprest J, Breysem L, Gratacos E, et al. Tracheal side effects following fetal endoscopic tracheal occlusion for severe congenital diaphragmatic hernia. Pediatr Radiol 2010;40:670-3. [Crossref] [PubMed]
- Jani J, Valencia C, Cannie M, et al. Tracheal diameter at birth in severe congenital diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Prenat Diagn 2011;31:699-704. [Crossref] [PubMed]
- Deprest J, Jani J, Van Schoubroeck D, et al. Current consequences of prenatal diagnosis of congenital diaphragmatic hernia. J Pediatr Surg 2006;41:423-30. [Crossref] [PubMed]
- Ruano R, Duarte SA, Pimenta EJ, et al. Comparison between fetal endoscopic tracheal occlusion using a 1.0-mm fetoscope and prenatal expectant management in severe congenital diaphragmatic hernia. Fetal Diagn Ther 2011;29:64-70. [Crossref] [PubMed]
- Ali K, Bendapudi P, Polubothu S, et al. Congenital diaphragmatic hernia-influence of fetoscopic tracheal occlusion on outcomes and predictors of survival. Eur J Pediatr 2016;175:1071-6. [Crossref] [PubMed]
- Belfort MA, Olutoye OO, Cass DL, et al. Feasibility and Outcomes of Fetoscopic Tracheal Occlusion for Severe Left Diaphragmatic Hernia. Obstet Gynecol 2017;129:20-9. [Crossref] [PubMed]
- Dhillon GS, Maskatia SA, Loar RW, et al. The impact of fetal endoscopic tracheal occlusion in isolated left-sided congenital diaphragmatic hernia on left-sided cardiac dimensions. Prenat Diagn 2018;38:812-20. [Crossref] [PubMed]
- Rodó C, Illescas T, Arévalo S, et al. Follow-up of fetuses with congenital diaphragmatic hernia: The quantitative lung index. Eur J Obstet Gynecol Reprod Biol 2018;225:22-5. [Crossref] [PubMed]
- Morandi A, Macchini F, Ophorst M, et al. Tracheal Diameter and Respiratory Outcome in Infants with Congenital Diaphragmatic Hernia Treated by Fetal Endoscopic Tracheal Occlusion. Fetal Diagn Ther 2019;46:296-305. [Crossref] [PubMed]
- Style CC, Olutoye OO, Belfort MA, et al. Fetal endoscopic tracheal occlusion reduces pulmonary hypertension in severe congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2019;54:752-8. [Crossref] [PubMed]
- Al-Maary J, Eastwood MP, Russo FM, et al. Fetal Tracheal Occlusion for Severe Pulmonary Hypoplasia in Isolated Congenital Diaphragmatic Hernia: A Systematic Review and Meta-analysis of Survival. Ann Surg 2016;264:929-33. [Crossref] [PubMed]
- Araujo Júnior E, Tonni G, Martins WP, et al. Procedure-Related Complications and Survival Following Fetoscopic Endotracheal Occlusion (FETO) for Severe Congenital Diaphragmatic Hernia: Systematic Review and Meta-Analysis in the FETO Era. Eur J Pediatr Surg 2017;27:297-305. [Crossref] [PubMed]
- Sacco A, Van der Veeken L, Bagshaw E, et al. Maternal complications following open and fetoscopic fetal surgery: A systematic review and meta-analysis. Prenat Diagn 2019;39:251-68. [Crossref] [PubMed]
- Grivell RM, Andersen C, Dodd JM. Prenatal interventions for congenital diaphragmatic hernia for improving outcomes. Cochrane Database Syst Rev 2015;CD008925 [Crossref] [PubMed]
- Basurto D, Russo FM, Van der Veeken L, et al. Prenatal diagnosis and management of congenital diaphragmatic hernia. Best Pract Res Clin Obstet Gynaecol 2019;58:93-106. [Crossref] [PubMed]
- Seravalli V, Jelin EB, Miller JL, et al. Fetoscopic tracheal occlusion for treatment of non-isolated congenital diaphragmatic hernia. Prenat Diagn 2017;37:1046-9. [Crossref] [PubMed]
- Sananès N, Regnard P, Mottet N, et al. Evaluation of a new balloon for fetal endoscopic tracheal occlusion in the nonhuman primate model. Prenat Diagn 2019;39:403-8. [Crossref] [PubMed]
- Basurto D, Sananes N, Russo FM, et al. Safety and efficacy fo the Smart tracheal occlusion balloon for congenital diaphragmatic hernia. Am J Obstet Gynecol 2020; [Epub ahead of print]. [Crossref]
- Luong C, Rey-Perra J, Vadivel A, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation 2011;123:2120-31. [Crossref] [PubMed]
- Russo FM, Toelen J, Eastwood MP, et al. Transplacental sildenafil rescues lung abnormalities in the rabbit model of diaphragmatic hernia. Thorax 2016;71:517-25. [Crossref] [PubMed]
- Russo FM, Benachi A, Van Mieghem T, et al. Antenatal sildenafil administration to prevent pulmonary hypertension in congenital diaphragmatic hernia (SToP-PH): study protocol for a phase I/IIb placenta transfer and safety study. Trials 2018;19:524. [Crossref] [PubMed]
- Russo FM, Mori da Cunha MG, Jimenez J, et al. Complementary Effect of Maternal Sildenafil and Fetal Tracheal Occlusion Improves Lung Development in the Rabbit Model of Congenital Diaphragmatic Hernia. Ann Surg 2020; [Epub ahead of print]. [Crossref] [PubMed]


